13.3
Impact Factor
Theranostics 2020; 10(15):6599-6614. doi:10.7150/thno.44226 This issue Cite
Research Paper
Targeting of Formyl Peptide Receptor 2 for in vivo imaging of acute vascular inflammation
1. Department of Chemistry, Imperial College London, Molecular Sciences Research Hub, White City, London, W12 0BZ, UK.
2. Department of Neurology, Louisiana State University Health Sciences Center-Shreveport, Shreveport, LA, 71130, USA.
3. Department of Molecular & Cellular Physiology, Louisiana State University Health Sciences Center-Shreveport, Shreveport, LA, 71130, USA.
4. Institute of Medical Biochemistry, Centre for Molecular Biology of Inflammation, University of Muenster, D-48149 Muenster, Germany.
5. Institute for Immunology, University of Muenster, D-48149 Muenster, Germany.
6. Department of Life Sciences, Brunel University London, Uxbridge, Middlesex, UB8 3PH, UK.
#Authors contributed equally
Abstract
Inflammatory conditions are associated with a variety of diseases and can significantly contribute to their pathophysiology. Neutrophils are recognised as key players in driving vascular inflammation and promoting inflammation resolution. As a result, neutrophils, and specifically their surface formyl peptide receptors (FPRs), are attractive targets for non-invasive visualization of inflammatory disease states and studying mechanistic details of the process.
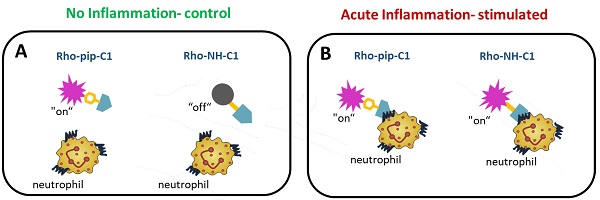
Methods: A small-molecule Formyl Peptide Receptor 2 (FPR2/ALX)-targeted compound was combined with two rhodamine-derived fluorescent tags to form firstly, a targeted probe (Rho-pip-C1) and secondly a targeted, pH-responsive probe (Rho-NH-C1) for in vivo applications. We tested internalization, toxicity and functional interactions with neutrophils in vitro for both compounds, as well as the fluorescence switching response of Rho-NH-C1 to neutrophil activation. Finally, in vivo imaging (fluorescent intravital microscopy [IVM]) and therapeutic efficacy studies were performed in an inflammatory mouse model.
Results: In vitro studies showed that the compounds bound to human neutrophils via FPR2/ALX without causing internalization at relevant concentrations. Additionally, the compounds did not cause toxicity or affect neutrophil functional responses (e.g. chemotaxis or transmigration). In vivo studies using IVM showed Rho-pip-C1 bound to activated neutrophils in a model of vascular inflammation. The pH-sensitive (“switchable”) version termed Rho-NH-C1 validated these findings, showing fluorescent activity only in inflammatory conditions.
Conclusions: These results indicate a viable design of fluorescent probes that have the ability to detect inflammatory events by targeting activated neutrophils.
Keywords: Inflammation, neutrophils, formyl peptide receptors, small-molecule imaging probes, intravital microscopy
 Global reach, higher impact
Global reach, higher impact