13.3
Impact Factor
Theranostics 2020; 10(14):6517-6529. doi:10.7150/thno.44274 This issue Cite
Research Paper
A rapid liquid biopsy of lung cancer by separation and detection of exfoliated tumor cells from bronchoalveolar lavage fluid with a dual-layer “PERFECT” filter system
1. Institute of Microelectronics, Peking University, Beijing, 100871, China;
2. Antimicrobial Resistance (AMR) and Critical Analytics for Manufacturing Personalized-Medicine (CAMP) IRG, Singapore-MIT Alliance for Research and Technology (SMART) Centre, Singapore, 138602, Singapore;
3. Department of Respiratory and Critical Care Medicine, Peking University First Hospital, Beijing, 100034, China;
4. Department of Geriatrics, Peking University First Hospital, Beijing, 100034, China;
5. Department of Pathology, Peking University First Hospital, Beijing, 100034, China;
6. National Key Laboratory of Science and Technology on Micro/Nano Fabrication, Beijing, 100871, China;
7. Frontiers Science Center for Nano-optoelectronics, Peking University, Beijing, 100871, China.
# The two authors contributed equally to this work.
Abstract
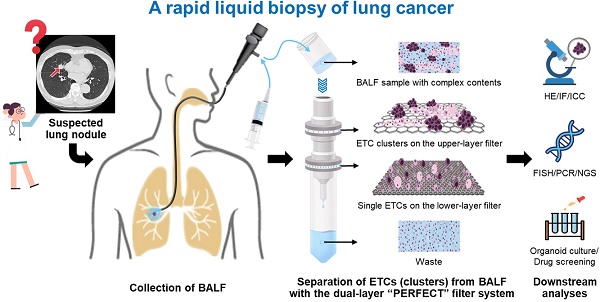
Separation and detection of exfoliated tumor cells (ETCs) from bronchoalveolar lavage fluid (BALF), namely the liquid biopsy of BALF, has been proved to be a valuable tool for the diagnosis of lung cancer. Herein, we established a rapid liquid biopsy of BALF based on a dual-layer PERFECT (precise, efficient, rapid, flexible, easy-to-operate, controllable and thin) filter system for the first time.
Methods: The dual-layer PERFECT filter system consists of an upper-layer filter with large micropores (feature size of 49.4 ± 0.5 μm) and a lower-layer filter with small micropores (9.1 ± 0.1 μm). The upper-layer filter contributes to the isolation of cell clusters and removal of mucus from BALF samples, meanwhile the lower-layer one targets for the separation of single ETCs. First, separation of 10000 spiked A549s (cultured lung cancer cells) from 10 mL clinical BALF samples (n=3) were performed to investigate the performance of the proposed system in rare cell separation. Furthermore, separation and detection of ETCs and ETC clusters from clinical BALF samples were performed with this system to test its efficacy and compared with the routine cytocentrifuge. The clinical BALF samples were collected from 33 lung cancer-suspected patients with visible lesions under bronchoscope. The final histopathological results showed that 20 samples were from lung cancer positive patients while the other 13 cases were from lung cancer negative patients.
Results: The recovery rate of spiked A549 cells from clinical BALF samples with the developed system (89.8 ± 5.2%) is significantly higher than that with the cytocentrifuge (13.6 ± 7.8%). In the preliminary clinical trial, although 33 clinical BALF samples with volume ranging from 6 mL to 18 mL showed greatly varied turbidity, filtrations could be finished within 3 min for 54.6% of samples (18/33), and 10 min at most for the rest. The dual-layer PERFECT filter system is proved to have a much higher sensitivity (80.0%, 95% CI: 55.7%-93.4%) than the routine cytocentrifuge (45.0%, 95% CI: 23.8%-68.0%), p=0.016 (McNemar test, two-tail). Moreover, the sensitivity of this platform is neither interfered by the variations of turbidity of the BALF samples, nor associated with the types of lung cancer.
Conclusions: The easy and rapid processing of BALF samples with varying volume and turbidity, competitive sensitivity and good versatility for different lung cancer types will make the established dual-layer PERFECT filter system a promising approach for the liquid biopsy of BALF. The high-performance BALF-based liquid biopsy will improve the cytopathological identification and diagnosis of lung cancer.
Keywords: lung cancer, liquid biopsy, exfoliated tumor cells (ETCs), bronchoalveolar lavage fluid (BALF), PERFECT filter
 Global reach, higher impact
Global reach, higher impact