13.3
Impact Factor
Theranostics 2020; 10(14):6384-6398. doi:10.7150/thno.45558 This issue Cite
Research Paper
Diethyldithiocarbamate-copper nanocomplex reinforces disulfiram chemotherapeutic efficacy through light-triggered nuclear targeting
1. State Key Laboratory of Environmental Chemistry and Ecotoxicology, Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences, Beijing, 100085, China.
2. State Key Laboratory for Physical Chemistry of Solid Surfaces, Collaborative Innovation Center of Chemistry for Energy Materials, and National & Local Joint Engineering Research Center for Preparation Technology of Nanomaterials, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen, 361005, China.
3. University of Chinese Academy of Sciences, Beijing, 100049, China.
#: These authors equally contribute to this work.
Abstract
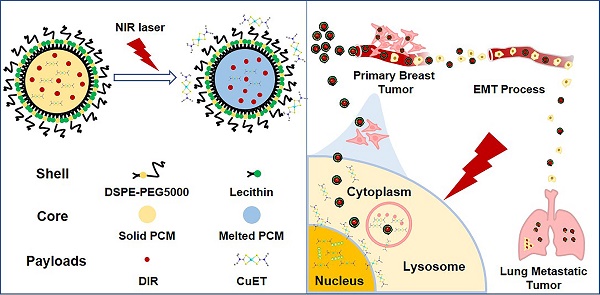
To circumvent the huge cost, long R&D time and the difficulty to identify the targets of new drugs, repurposing the ones that have been clinically approved has been considered as a viable strategy to treat different diseases. In the current study, we outlined the rationale for repurposing disulfiram (DSF, an old alcohol-aversion drug) to treat primary breast cancer and its metastases.
Methods: To overcome a few shortcomings of the individual administration of DSF, such as the dependence on copper ions (Cu2+) and limited capability in selective targeting, we here artificially synthesized the active form of DSF, diethyldithiocarbamate (DTC)-Cu complex (CuET) for cancer therapeutics. To achieve a greater efficacy in vivo, smart nanomedicines were devised through a one-step self-assembly of three functional components including a chemically stable and biocompatible phase-change material (PCM), the robust anticancer drug (CuET) and a near-infrared (NIR) dye (DIR), namely CuET/DIR NPs. A number of in vitro assays were performed including the photothermal efficacy, light-triggered drug release behavior, nuclear localization, DNA damage and induction of apoptosis of CuET/DIR NPs and molecular mechanisms underlying CuET-induced repression on cancer metastatic behaviors. Meanwhile, the mice bearing 4T1-LG12-drived orthotopic tumors were employed to evaluate in vivo biodistribution and anti-tumor effect of CuET/DIR NPs. The intravenous injection model was employed to reflect the changes of the intrinsic metastatic propensity of 4T1-LG12 cells responding to CuET/DIR NPs.
Results: The rationally designed nanomedicines have self-traceability for bioimaging, long blood circulation time for enhanced drug accumulation in the tumor site and photo-responsive release of the anticancer drugs. Moreover, our data unearthed that CuET/DIR nanomedicines behave like “Trojan horse” to transport CuET into the cytoplasm, realizing substantial intracellular accumulation. Upon NIR laser irradiation, massive CuET would be triggered to release from the nanomedicines and reach a high local concentration towards the nucleus, where the pro-apoptotic effects were conducted. Importantly, our CuET/DIR nanomedicines revealed a pronounced capability to leash breast cancer metastases through inhibition on EMT. Additionally, these nanomedicines showed great biocompatibility in animals.
Conclusion: These combined data unearthed a remarkably enhanced tumor-killing efficacy of our CuET nanomedicines through nuclear targeting. This work may open a new research area of repurposing DSF as innovative therapeutic agents to treat breast cancer and its metastases.
Keywords: diethyldithiocarbamate, DSF-Cu nanocomplex, light-triggered nuclear targeting, metastasis, epithelial-mesenchymal transition
 Global reach, higher impact
Global reach, higher impact