13.3
Impact Factor
Theranostics 2020; 10(13):6024-6034. doi:10.7150/thno.39554 This issue Cite
Research Paper
Instant labeling of therapeutic cells for multimodality imaging
1. Department of Radiology, Molecular Imaging Program at Stanford, Stanford University, CA, 94305, USA
2. Department of Radiation Oncology, Stanford University, CA, 94305, USA
3. Department of Diagnostic and Interventional Radiology, University Medical Center of the Johannes Gutenberg-University Mainz, 55131 Mainz, Germany
4. Department of Biomedical Engineering, Georgia Institute of Technology, Atlanta, GA, 30332, USA
5. Department of Pediatrics, Stanford University, CA, 94305, USA
# These authors contributed equally.
Abstract
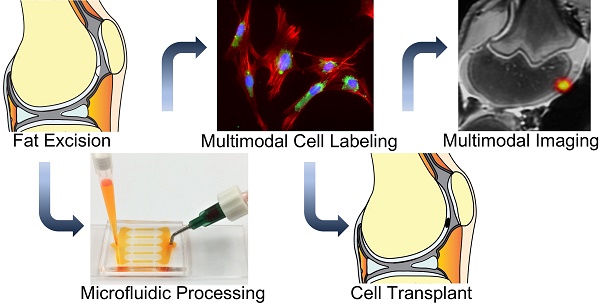
Autologous therapeutic cells are typically harvested and transplanted in one single surgery. This makes it impossible to label them with imaging biomarkers through classical transfection techniques in a laboratory. To solve this problem, we developed a novel microfluidic device, which provides highly efficient labeling of therapeutic cells with imaging biomarkers through mechanoporation.
Methods: Studies were performed with a new, custom-designed microfluidic device, which contains ridges, which compress adipose tissue-derived stem cells (ADSCs) during their device passage. Cell relaxation after compression leads to cell volume exchange for convective transfer of nanoparticles and nanoparticle uptake into the cell. ADSCs were passed through the microfluidic device doped with iron oxide nanoparticles and 18F-fluorodeoxyglucose (FDG). The cellular nanoparticle and radiotracer uptake was evaluated with DAB-Prussian blue, fluorescent microscopy, and inductively coupled plasma spectrometry (ICP). Labeled and unlabeled ADSCs were imaged in vitro as well as ex vivo in pig knee specimen with magnetic resonance imaging (MRI) and positron emission tomography (PET). T2 relaxation times and radiotracer signal were compared between labeled and unlabeled cell transplants using Student T-test with p<0.05.
Results: We report significant labeling of ADSCs with iron oxide nanoparticles and 18F-FDG within 12+/-3 minutes. Mechanoporation of ADSCs with our microfluidic device led to significant nanoparticle (> 1 pg iron per cell) and 18F-FDG uptake (61 mBq/cell), with a labeling efficiency of 95%. The labeled ADSCs could be detected with MRI and PET imaging technologies: Nanoparticle labeled ADSC demonstrated significantly shorter T2 relaxation times (24.2±2.1 ms) compared to unlabeled cells (79.6±0.8 ms) on MRI (p<0.05) and 18F-FDG labeled ADSC showed significantly higher radiotracer uptake (614.3 ± 9.5 Bq / 1×104 cells) compared to controls (0.0 ± 0.0 Bq/ 1×104 cells) on gamma counting (p<0.05). After implantation of dual-labeled ADSCs into pig knee specimen, the labeled ADSCs revealed significantly shorter T2 relaxation times (41±0.6 ms) compared to unlabeled controls (90±1.8 ms) (p<0.05).
Conclusion: The labeling of therapeutic cells with our new microfluidic device does not require any chemical intervention, therefore it is broadly and immediately clinically applicable. Cellular labeling using mechanoporation can improve our understanding of in vivo biodistributions of therapeutic cells and ultimately improve long-term outcomes of therapeutic cell transplants.
Keywords: mechanoporation, microfluidic device, iron oxide nanoparticles, 18F-FDG, in vivo cell tracking
 Global reach, higher impact
Global reach, higher impact