13.3
Impact Factor
Theranostics 2020; 10(13):5815-5828. doi:10.7150/thno.44334 This issue Cite
Research Paper
The tumor targeting performance of anti-CD166 Probody drug conjugate CX-2009 and its parental derivatives as monitored by 89Zr-immuno-PET in xenograft bearing mice
1. Amsterdam UMC, Vrije Universiteit, Dept. Radiology and Nuclear Medicine, Tracer Center Amsterdam, De Boelelaan 1117, Amsterdam, The Netherlands.
2. CytomX Therapeutics, Inc., 151 Oyster Point Blvd., Suite 400, South San Francisco, CA 94080, USA.
*Contributed equally
Abstract
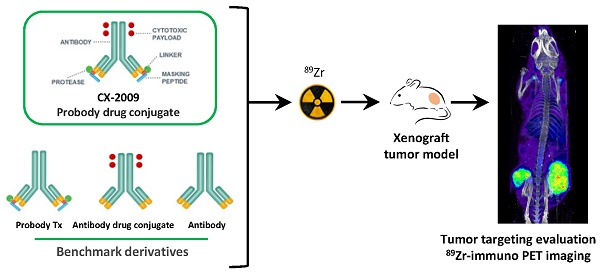
Probody® therapeutics are recombinant masked monoclonal antibody (mAb) prodrugs that become activated by proteases present in the tumor microenvironment. This makes them attractive for use as Probody drug conjugates (PDCs). CX-2009 is a novel PDC with the toxic drug DM4 coupled to an anti-CD166 Probody therapeutic. CD166 is overexpressed in multiple tumor types and to a lesser extent by healthy tissue.
Methods: The tumor targeting potential of CX-2009 was assessed by performing 89Zr-immuno-PET/biodistribution/therapy studies in a CD166-positive H292 lung cancer mouse model. Head-to-head comparisons of CX-2009 with the Probody therapeutic without DM4 (CX-191), the unmasked antibody drug conjugate (ADC) CX-1031, and the parental mAb CX-090 were performed. All constructs were 89Zr labeled in a GMP compliant way, administered at 10, 110, or 510 µg, and ex vivo biodistribution was assessed at 24, 72, and 168 hours post-injection.
Results: Comparable biodistribution was observed for all constructs, confirmed with PET/CT. Tumors showed the highest uptake: 21.8 ± 2.3 ([89Zr]Zr-CX-2009), 21.8 ± 5.0 ([89Zr]Zr‑CX-191), 18.7 ± 2.5 ([89Zr]Zr-CX-1031) and 20.8 ± 0.9 %ID/g ([89Zr]Zr-CX-090) at 110 µg injected. Increasing the dose to 510 µg resulted in lower tumor uptake and higher blood levels for all constructs, suggesting receptor saturation. In addition, CX-2009 and CX-1031 showed similar therapeutic potential.
Conclusions: CX-2009 is optimally capable of targeting CD166-expressing tumors when compared with its derivatives, implying that enzymatic activation inside the tumor, required to allow CD166 binding, does not limit tumor targeting. Because CX-2009 does not bind to mouse CD166, however, reduced targeting of healthy organs should be confirmed in ongoing clinical 89Zr-immuno-PET studies.
 Global reach, higher impact
Global reach, higher impact