13.3
Impact Factor
Theranostics 2020; 10(13):5802-5814. doi:10.7150/thno.44772 This issue Cite
Research Paper
89Zr-PET imaging of DNA double-strand breaks for the early monitoring of response following α- and β-particle radioimmunotherapy in a mouse model of pancreatic ductal adenocarcinoma
1. Department of Radiology, Memorial Sloan Kettering Cancer Center, NY, USA
2. CRUK/MRC Oxford Institute of Radiation Oncology, Department of Oncology, University of Oxford, Oxford, UK
3. School of Natural and Environmental Sciences, Newcastle University, Newcastle upon Tyne, UK
4. Radiochemistry and Molecular Imaging Probes Core, Memorial Sloan Kettering Cancer Center, New York, NY, USA
5. Weill Cornell Medical College, New York, NY, USA
6. Molecular Pharmacology Program, Memorial Sloan Kettering Cancer Center, New York, NY, USA
Abstract
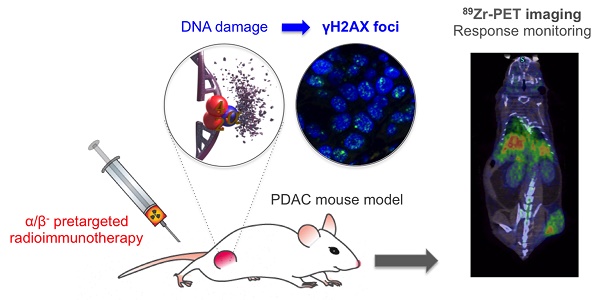
Rationale: The evaluation of early treatment response is critical for patient prognosis and treatment planning. When the current methods rely on invasive protocols that evaluate the expression of DNA damage markers on patient biopsy samples, we aim to evaluate a non-invasive PET imaging approach to monitor the early expression of the phosphorylated histone γH2AX in the context of pancreatic cancer targeted radionuclide therapy. Pancreatic ductal adenocarcinoma has a poor patient prognosis due to the absence of curative treatment for patients with advanced disease. There is therefore a critical need for the fast clinical translation of new therapeutic options. In line with these observations, our group has been focusing on the development of radiotheranostic agents based on a fully human monoclonal antibody (5B1) with exceptional affinity for CA19.9, an antigen overexpressed in PDAC. Two on-going clinical trials resulted from these efforts, one with 89Zr (diagnosis) and one with 177Lu (β-particle therapy). More recently, we successfully developed and evaluated in PDAC mouse models a targeted α-therapy strategy with high clinical translation potential. We aim to expedite the clinical translation of the developed radioimmunotherapy approaches by investigating the early therapeutic response and effect of radiation therapy in a PDAC mouse model via PET imaging.
Methods: Mice bearing BxPC3 tumor xenografts were treated with α- and β-particle pretargeted radioimmunotherapy (PRIT), external beam radiotherapy (EBRT), or sham-treated (vehicle). The phosphorylated histone γH2AX produced as a response to DNA double strand breaks was quantified with the PET radiotracer, [89Zr]Zr-DFO-anti-γH2AX-TAT.
Results: PET imaging studies in BxPC3 PDAC mouse models demonstrated increased uptake of [89Zr]Zr-DFO-anti-γH2AX-TAT (6.29 ± 0.15 %IA/g) following β-PRIT in BxPC3 PDAC xenografts as compared to the saline control group (4.58 ± 0.76 %IA/g) and EBRT control group (5.93 ± 0.76 %IA/g). Similarly, significantly higher uptake of [89Zr]Zr-DFO-anti-γH2AX-TAT was observed in tumors of the 225Ac-PRIT and EBRT (10 Gy) cohorts (7.37 ± 1.23 and 6.80 ± 1.24 %IA/g, respectively) compared to the negative control cohort (5.08 ± 0.95 %IA/g). Ex vivo γH2AX immunohistochemistry and immunofluorescence analysis correlated with in vivo 89Zr-anti-γH2AX PET/CT imaging with increased γH2AX positive cell and γH2AX foci per cell in the treated cohorts. When α-PRIT resulted in prolonged overall survival of treated animals (107.5 days) as compared to β-PRIT (73.0 days), no evidence of difference in [89Zr]Zr-DFO-anti-γH2AX-TAT uptake at the tumor site was observed, highlighting that DNA damage is not the sole radiobiology paradigm and that off-targeted (bystander) effects should be considered.
Conclusions: PET imaging studies with [89Zr]Zr-DFO-anti-γH2AX-TAT following α- and β-particle PRIT in a BxPC3 PDAC subcutaneous xenograft mouse model allowed the monitoring of tumor radiobiological response to treatment.
Keywords: Radioimmunotherapy, DNA damage, γH2AX, PET, pancreatic cancer
 Global reach, higher impact
Global reach, higher impact