13.3
Impact Factor
Theranostics 2020; 10(12):5169-5180. doi:10.7150/thno.43231 This issue Cite
Research Paper
Selective delivery of T22-PE24-H6 to CXCR4+ diffuse large B-cell lymphoma cells leads to wide therapeutic index in a disseminated mouse model
1. Biomedical Research Institute Sant Pau (IIB-Sant Pau), Hospital de la Santa Creu i Sant Pau, Barcelona Spain.
2. Josep Carreras Leukaemia Research Institute (IJC), Barcelona, Spain.
3. CIBER de Bioingeniería Biomateriales y Nanomedicina (CIBER-BBN), Barcelona, Spain.
4. Institute of Biotechnology and Biomedicine (IBB), Universitat Autònoma de Barcelona, Barcelona, Spain.
5. Department of Genetics and Microbiology, Universitat Autònoma de Barcelona, Barcelona, Spain.
6. Department of Hematology, Hospital de la Santa Creu i Sant Pau, Barcelona, Spain.
Abstract
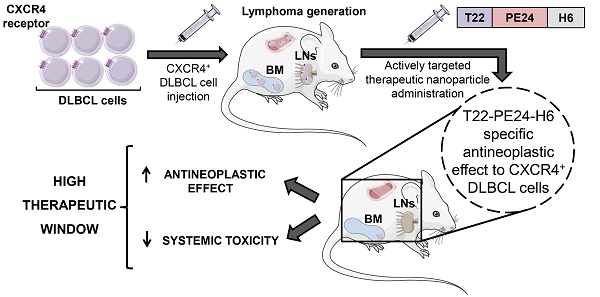
Background: Novel therapeutic strategies are urgently needed to reduce relapse rates and enhance survival in Diffuse Large B-Cell Lymphoma (DLBCL) patients. CXCR4-overexpressing cancer cells are good targets for therapy because of their association with dissemination and relapse in R-CHOP treated DLBCL patients. Immunotoxins that incorporate bacterial toxins are potentially effective in treating haematological neoplasias, but show a narrow therapeutic index due to the induction of severe side effects. Therefore, when considering the delivery of these toxins as cancer therapeutics, there is a need not only to increase their uptake in the target cancer cells, and their stability in blood, but also to reduce their systemic toxicity. We have developed a therapeutic nanostructured protein T22-PE24-H6 that incorporates exotoxin A from Pseudomonas aeruginosa, which selectively targets lymphoma cells because of its specific interaction with a highly overexpressed CXCR4 receptor (CXCR4+) in DLBCL.
Methods: T22-PE24-H6 cytotoxicity and its dependence on the CXCR4 receptor were evaluated in DLBCL cell lines using cell viability assays. Different in vitro experiments (mitochondrial membrane potential, Western Blot, Annexin V and DAPI staining) were conducted to determine T22-PE24-H6 cell death mechanisms. In vivo imaging and therapeutic effect studies were performed in a disseminated DLBCL mouse model that mimics organ infiltration in DLBCL patients. Finally, immunohistochemistry and histopathology analyses were used to evaluate the antineoplastic effect and systemic toxicity.
Results: In vitro, T22-PE24-H6 induced selective cell death of CXCR4+ DLBCL cells by activating the apoptotic pathway. In addition, repeated T22-PE24-H6 intravenous administration in a CXCR4+ DLBCL-disseminated mouse model showed a significant reduction of lymphoma burden in organs clinically affected by DLBCL cells (lymph nodes and bone marrow). Finally, we did not observe systemic toxicity associated to the nanoparticle treatment in non-DLBCL-infiltrated organs.
Conclusion: We have demonstrated here a potent T22-PE24-H6 antineoplastic effect, especially in blocking dissemination in a CXCR4+ DLBCL model without associated toxicity. Thereby, T22-PE24-H6 promises to become an effective alternative to treat CXCR4+ disseminated refractory or relapsed DLBCL patients.
Keywords: targeted nanoparticle, CXCR4 receptor, PE24 exotoxin, DLBCL
 Global reach, higher impact
Global reach, higher impact