13.3
Impact Factor
Theranostics 2020; 10(11):5011-5028. doi:10.7150/thno.42742 This issue Cite
Research Paper
Extracellular vesicles from human umbilical cord blood plasma modulate interleukin-2 signaling of T cells to ameliorate experimental autoimmune encephalomyelitis
1. Department of Microbiology, College of Medicine, The Catholic University of Korea, Seoul, Republic of Korea;
2. Department of Biomedicine and Health Sciences, College of Medicine, The Catholic University of Korea, Seoul, Republic of Korea;
3. Catholic Hematopoietic Stem Cell Bank, College of Medicine, The Catholic University of Korea, Seoul, Republic of Korea.
Abstract
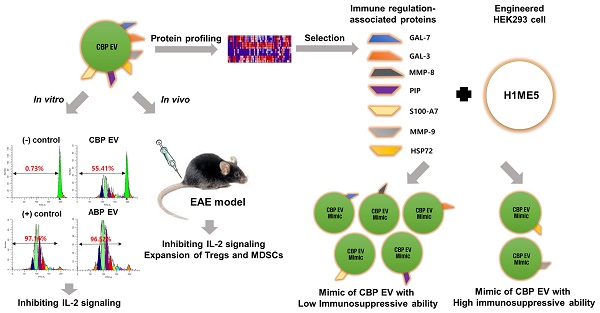
Human umbilical cord blood (UCB) cell-derived extracellular vesicles (EV) reportedly play immunosuppressive roles; however, UCB plasma-derived extracellular vesicles (CBP EVs) remain poorly studied. We examined the immunosuppressive potential of CBP EVs compared to that of adult blood plasma-derived extracellular vesicles (ABP EVs) in vitro and constructed an experimental autoimmune encephalomyelitis (EAE) model.
Methods: CBP EVs were isolated by ultracentrifugation and their proteomic profiling was performed using the high-resolution liquid chromatography with tandem mass spectrometry. Human T lymphocytes or mouse splenocytes labeled with carboxyfluorescein succinimidyl ester were incubated with CBP EV to measure the immunosuppressive function of CBP EV. The effect on T-cell polarization was analyzed by flow cytometry and enzyme-linked immunospot assay. The matrix metalloproteinase (MMP) function in CBP EV was specifically inhibited using a chemical inhibitor. The efficacy of CBP EVs in the EAE mouse model was determined by scoring the symptoms and analyzing cell phenotype and cytokines using mouse splenocytes. We generated genetically engineered artificial EVs using HLA/MIC-null HEK293T (H1ME-5) cell line to characterize the immunosuppressive effect of CBP EV.
Results: CBP EVs primarily inhibited the proliferation of T cells by reducing the production of IL-2. Specifically, CBP EV-derived matrix metallopeptidase cleaved the IL-2 receptor α (CD25) on the surface of activated T cells, consequently downregulating IL-2 signaling in response to IL-2R engagement. Although the inhibition of MMP activity in CBP EVs abrogated CD25 cleavage and restored IL-2 production in activated T cells, the immunosuppressive response was not fully recovered. Thus, we further analyzed changes in immunosuppressive cells such as regulatory T cells and bone marrow-derived suppressor cells by CBP EV. Further, GAL-3, GAL-7, S100-A7, MMP-9, MMP-8, HSP-72, and PIP were highly enriched in CBP EV-mimics in which they served as pivotal mediators of CBP EV-induced immunosuppressive effects. Therefore, we generated genetically engineered GAL-3, GAL-7, S100-A7, MMP-9, MMP-8, HSP-72, and PIP-EVs using HLA/MIC-null HEK293T cells to characterize the immunosuppressive effect of these molecules. Among these, MMP-9 and HSP-72-enriched EVs showed the most significant T cell immunosuppression.
Conclusion: CBP EVs inhibited T cell proliferation and EAE development by modulating IL-2 signaling and immunosuppressive cell fate. CBP EVs contain critical components for immunosuppression and that CBP EV mimics, specifically those expressing MMP-9 and HSP-72, may offer a novel promising strategy for the treatment of various autoimmune diseases.
Keywords: interleukin-2 (IL-2) signaling, matrix metalloproteinase-9 (MMP-9), regulatory T cell (Treg), umbilical cord blood plasma-derived extracellular vesicles (CBP EVs), experimental autoimmune encephalomyelitis (EAE)
 Global reach, higher impact
Global reach, higher impact