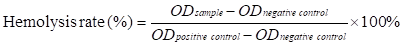13.3
Impact Factor
Theranostics 2020; 10(11):4795-4808. doi:10.7150/thno.42922 This issue Cite
Research Paper
pH responsive superporogen combined with PDT based on poly Ce6 ionic liquid grafted on SiO2 for combating MRSA biofilm infection
1. Department of pharmacy, Air Force Medical University, Xi'an, 710032, Shaanxi Province, China
2. Department of physics, Northwest University of Technology, Xi'an, 710032, Shaanxi Province, China
3. Department of Thoracic Surgery, Tangdu Hospital, Air Force Medical University, Xi'an, 710032, Shaanxi Province, China
*These authors contributed equally to this work
Received 2019-12-10; Accepted 2020-3-5; Published 2020-3-26
Abstract
Background: Biofilm infection caused by multidrug-resistant bacteria is difficult to eradicate by conventional therapies. Photodynamic therapy (PDT) is an effective antibacterial method for fighting against biofilm infection. However, the blocked photosensitizers outside of biofilm greatly limit the efficacy of PDT.
Methods: Herein, a novel acid-responsive superporogen and photosensitizer (SiO2-PCe6-IL) was developed. Because of the protonation of the photosensitizer and the high binding energy of the polyionic liquid, SiO2-PCe6-IL changed to positive SiO2-PIL+ in an acidic microenvironment of biofilm infection. SiO2-PIL+ could combine with negatively charged extracellular polymeric substances (EPS) and create holes to remove the biofilm barrier. To strengthen the interaction between SiO2-PIL+ and EPS, SiO2-PIL+ of high charge density was prepared by grafting the high-density initiation site of ATRP onto the surface of the SiO2 base.
Results: Due to the rapid protonation rate of COO- and the strong binding energy of SiO2-PIL+ with EPS, SiO2-PCe6-IL could release 90% of Ce6 in 10 s. With the stronger electrostatic and hydrophobic interaction of SiO2-PIL+ with EPS, the surface potential, hydrophobicity, adhesion and mechanical strength of biofilm were changed, and holes in the biofilm were created in 10 min. Combining with the release of photosensitizers and the porous structure of the biofilm, Ce6 was efficiently concentrated in the biofilm. The in vitro and in vivo antibacterial experiments proved that SiO2-PCe6-IL dramatically improved the PDT efficacy against MRSA biofilm infection.
Conclusion: These findings suggest that SiO2-PCe6-IL could rapidly increase the concentration of photosensitizer in biofilm and it is an effective therapy for combating biofilm infection.
Keywords: pH-responsive, polyionic liquids, superporogen, photosensitizer, SiO2, MRSA biofilm
Introduction
It is well known that bacteria shielded by biofilm are difficult to eradicate [1-3]. Once the biofilm forms, the resistance of the bacteria is increased by 1000~1500 times versus that of individual bacteria [4, 5]. Especially for the emergence and rapid spread of multidrug-resistant pathogens, biofilm infection is difficult to treat with conventional antibiotics [6, 7]. Hence, it is urgent to develop new therapeutic agents or strategies against drug-resistant bacterial biofilm infection.
Photodynamic therapy (PDT) based on photosensitizers to generate ROS through type I and type II oxidative reaction damages the structure and function of the biomolecule [8]. Compared with antibiotics, photosensitizers work through a multi-targeted oxidation mechanism against it which is impossible to develop resistance, and they also have excellent inhibitory activity against drug-resistant bacteria [9-11]. However due to the electrostatic repulsion between photosensitizers and EPS (the main negative components in biofilm) [12], most photosensitizers are difficultly concentrated in biofilm. Because 1O2 exhibits a short diffusion distance (approximately 10 nm) and short lifetime (3.5 μs) [13,14], the photosensitizers irradiated outside of the biofilm access the thick layer of biofilm (approximately 40 µM) with difficulty [15] and cannot kill the bacteria in the biofilm. In recent years, multifunctional photodynamic antibacterial systems have been used to reduce the repulsive interaction between the photosensitizers and EPS. For example, cationic polymers have been used to neutralize the negatively charged EPS and weaken the repulsion [16, 17]. However, the photosensitizers connected with cationic polymers via covalent bonding are immobilized on the EPS, and the lethal photosensitization occurs mainly in the outermost layers of biofilm [18]. The efficacy of PDT against biofilm has not been improved.
Due to the encapsulation of EPS, the infection site of biofilm is hypoxia, and the anaerobic glycolysis increase markedly. This result in the acidic microenvironment of the biofilm infection [19]. So, the acid-sensitive covalent bond is used to solve the problem of photosensitizer release [20, 21]. However, our previous research found that the traditional acid sensitive bond such as hydrazone takes approximately 24-48 h to break above 80%. Photosensitizers are difficult to release. Unreleased photosensitizers cannot penetrate through biofilm and concentrate in biofilm. Because the bacteria grow rapidly and can reproduce in 10-20 min [22], the low enrichment efficiency of photosensitizers in biofilm easily miss the best treatment time. Therefore, a new strategy should be explored to overcome the release rate of photosensitizers.
Ionic liquids comprise cations and non- covalently connected anions [23, 24]. Compared with the strong covalent bonds, the unique interaction of ionic liquids is relatively weak and more likely to exhibit rapid dissociation behavior [25, 26]. Thus the carboxyl groups of photosensitizers as the anion of ionic liquids could solve the release problem of photosensitizers caused by the linkage of covalent bonds. More importantly, as a “designed” substance, the structure of cations can be designed to have a specific function [27]. For instance, changing the carbon chain length at the N3 position of imidazolium cation is expected to destroy the biofilm integrity [28, 29]. Therefore, the combination of ionic liquids and photosensitizers can not only solve the release problem but also eliminate the biofilm barrier and then rapidly increase the concentration of photosensitizers in the biofilm.
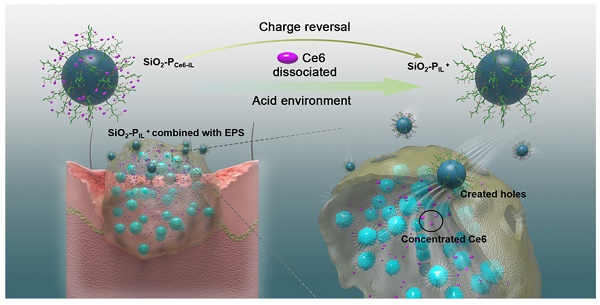
In this work, Ce6 with three COO- as the anions and 1-vinyl imidazole with dodecyl as the cation and the pH-responsive Ce6 ionic liquid (Ce6-IL) were assembled by an anion exchange reaction. SiO2 nanoparticles were introduced to graft different concentration initiation sites of atom transfer radical polymerization (ATRP) and control the density of Ce6-IL polymers. In the physiological environment, the hole-forming ability of SiO2-PCe6-IL was shielded to reduce the damage to normal tissue. In the acidic environment of biofilm infection, Ce6 was protonated and released. Meanwhile, the SiO2-PCe6-IL reversed to SiO2-PIL+ for bonding with negatively charged EPS and was hammered into biofilm to create holes (Figure 1). For maximum punch capacity, SiO2-PCe6-IL of high charge density was prepared. After the Ce6 concentrated in biofilm, the illumination of 660 nm was used to produce ROS to kill MRSA.
Results and Discussion
The selection of anion and cation
The photosensitizer Ce6, which has three COO- and can be protonated in an acidic environment, was selected as the anion. However, because everything inside the biofilm occurs in a gradient (nutrients, oxygen, and pH itself), the biofilm did not have a unique pH and not all of three COO- could be protonated in weak acid environment. Other strategies should be used to ensure that photosensitizers can be released under both weakly and strongly acidic conditions. In this work, the cation was designed to enhance the interaction between cations and EPS. When the designed cation was combined with EPS, the stronger interaction made it dissociate with Ce6 and then accelerated the release of Ce6. The structure of the cation was designed as follows: the 1-viny-3-dodecyl-imidazole (IL) that could bond with EPS and destroy biofilm integrity [30, 31] was selected as the cation. To estimate the interaction energy of 1-vinyl-3-dodecyl imidazole and EPS, the binding energy (ΔΕ) of 1-vinyl-3-dodecyl imidazole and polysaccharide poly-β-1, 6-N-acetylglucosamine (PNAG) [32], which is the main factor of EPS that maintains the integrity of the biofilm structure, was measured by molecular dynamic simulation (Figure S1). As shown in Table 1, the ΔΕ of 1-vinyl-3-dodecyl imidazole with PNAG (ΔΕ1) was -6.839135 Kcal/mol, and when the 1-vinyl-3-dodecyl imidazole was polymerized to form polycation (PIL+), the ΔΕ2 of PIL+ with PNAG was -23.442899 Kcal/mol and increased significantly. The stronger interaction of PIL+ and PNAG will accelerate Ce6 release even in a weakly acidic environment (pH less than pKa of Ce6) and can provide a great destructive power in destroying the structure of biofilm.
The energy and the binding energy (ΔE) of PIL+ with PNAG
| E (Kcal·mol-1) | ΔE (Kcal·mol-1) | |
|---|---|---|
| IL+ PIL+ | 51.210512 135.791181 | |
| PNAG | 112.303170 | |
| IL+ with PNAG | 156.674847 | -6.839135 [A] |
| PIL+ with PNAG | 236.882540 | -23.442899 [B] |
[A] ΔE1 = EIL+-PNAG - EIL+- EPNAG. [B] ΔE2 = EPIL+-PNAG - EPIL+- EPNAG.
Synthesis and characterization of Ce6-IL
As reported, the pH of the MRSA biofilm was approximately 5.5, 5.0 and even lower [33]. Thus, Chlorin e6 (Ce6) with three COOH was used as the model. Under the alkaline conditions, the three COOH of Ce6 changed to COO- and were assembled into Ce6-IL with 1-vinyl-3-dodecyl-imidazole (Figure S2). Because of the different modifications, the ionization constants (pKa) of Ce6 were varied. For example, the pKa of Ce6 alone was 4.4, 4.7, and 4.8, respectively. After conjugation with aptamers, the pKa of three Ce6 would occur between 6.5 and 8.5 [34]. In this work, when Ce6 was assembled with imidazole ionic liquid (Ce6-IL), the pKa was 4.6, 5.7 and 6.7, respectively (Figure S3). The other characterization of Ce6-IL has been shown in our previous research [35].
(A) Schematic illustrating the synthetic route and responsive properties of SiO2-PCe6-IL. (B) The SiO2-PIL+ bonded with negatively charged EPS and created holes in the biofilm and then the Ce6 was concentrated.
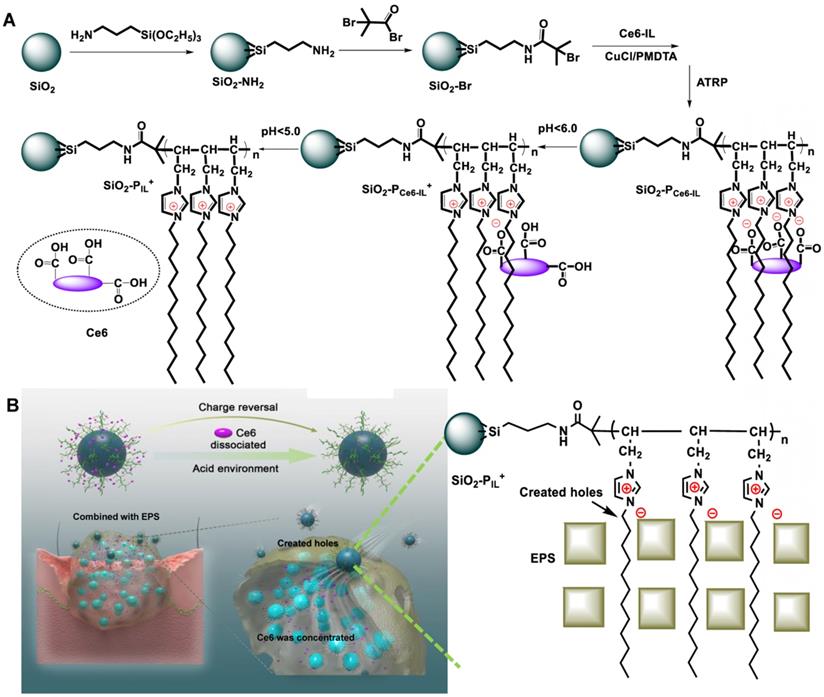
Synthesis of SiO2-PCe6-IL
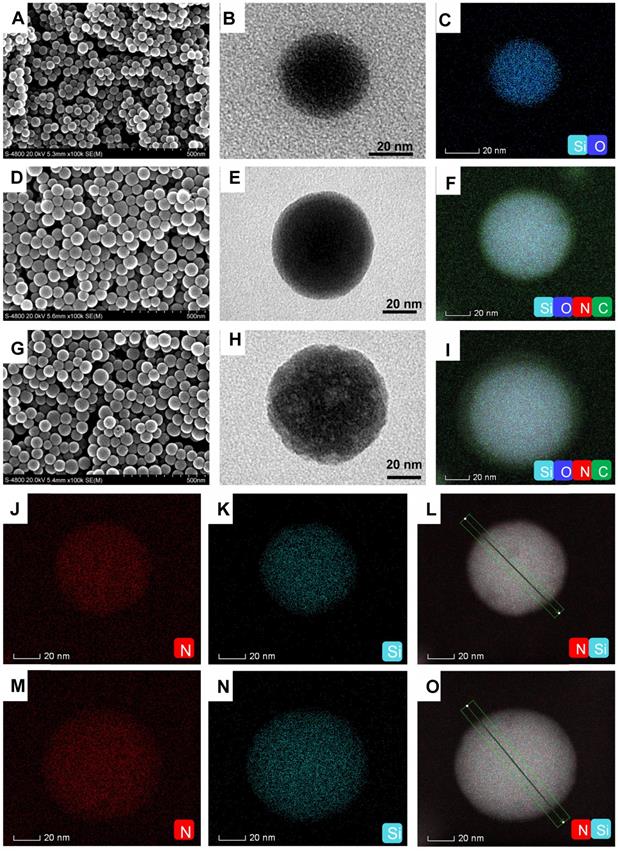
The computer simulation result showed that the polymerized Ce6-IL could strengthen the interaction of 1-vinyl-3-dodecyl imidazole and PNAG. Furthermore, because the chain length and positive charge density of polycations had an important influence on antimicrobial properties [36, 37], PCe6-IL of the different chain lengths and charge densities was grafted onto the SiO2 by regulating the concentration of the reactive sites (Br) of ATRP. The grafting content of Br on the SiO2 was 1.07% and 7.52% (Figure S4). After adding Ce6-IL and initiator CuCl, the site-specific in situ polymerization was induced. Because the same molar of monomer (Ce6-IL) was used, fewer initiation sites of Br on the SiO2 (1.07%) will lead to low charged density PCe6-IL1 with long polymer chains on the SiO2 (SiO2-PCe6-IL1). In contrast, high charged density PCe6-IL2 with short polymer chains on the SiO2 (SiO2-PCe6-IL2) were prepared by highly concentrated Br (7.52%). The SEM and TEM results showed that the size of SiO2 was approximately 40 nm (Figure 2 A, B, C). After polymerization, the size of SiO2-PCe6-IL1 and SiO2-PCe6-IL2 was approximately 60 (Figure 2 D, E, F) and 70 nm (Figure 2 G, H, I). The dynamic light scattering (DLS) results indicated that SiO2-PCe6-IL1 and SiO2-PCe6-IL2 have excellent stability in PBS (Figure S5). Although fewer reactive sites would result in a longer polymer chain and larger particle size, the size of SiO2-PCe6-IL1 was smaller than SiO2-PCe6-IL2. This may be caused by the longer polycationic polymer chains partly entwined with SiO2 nanoparticles.
The element analysis showed that the N of PCe6-IL appeared on the SiO2 after polymerization. The location of the Si and N was further analyzed by spherical aberration corrected transmission electron microscope (ACTEM, Figure 2J, L, N, O). The result showed that the N was on the surface of SiO2 (Figure 2M, P and Figure S6).
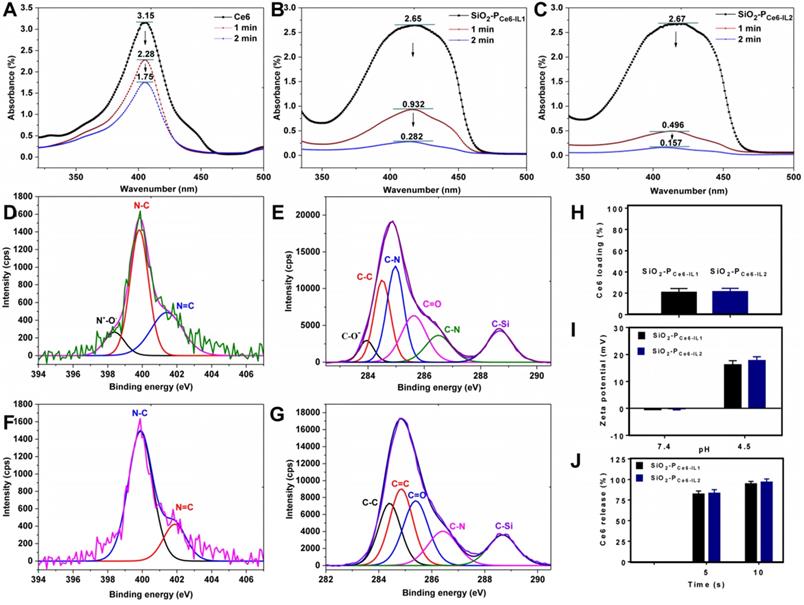
The production of 1O2. Because the production of 1O2 played an important role in PDT, the 1O2 production of SiO2-PCe6-IL was detected by 1, 3-diphenylisobenzofuran (DPBF). As the 1O2 can irreversibly oxidize the conjugated structure of DPBF, the reducing absorption band of DPBF corresponded with the 1O2 generation [38]. High 1O2 generation led to a greater decrease in ultraviolet absorption of DPBF at 410 nm. The DPBF consumption of SiO2-PCe6-IL was greater than that of Ce6 (Figure 3A, B, C). However, the ultraviolet absorption of Ce6 and SiO2-PCe6-IL at 410 nm was not decreased significantly after illumination for 1 and 2 min (Figure S7). The consumption of DPBF was mainly caused by the generation of 1O2. The high 1O2 generation efficiency may be caused by polyionic liquids providing a special solvent environment and could improve the stability of the photosensitive structure [39]. Due to the short lifetime of 1O2, the rapid and massive 1O2 was expected to significantly improve the efficacy of PDT. Compared with SiO2-PCe6-IL2, the 1O2 generation of SiO2-PCe6-IL1 was lower (Figure 3B, C). This may also be caused by the entanglement of long-chained PCe6-IL1 with SiO2 which prevented 1O2 from diffusing out of the polymer shell of SiO2-PCe6-IL1 and made it undetectable (the shell thickness was 20 nm, and the diffusion distance of 1O2 was only approximately 10 nm). More 1O2 production made SiO2-PCe6-IL2 have more oxidation capacity to combat biofilm infection.
The rapid acid responsive ability
The XPS was used to examine the chemical species of cation-anion bond (N+-O-) and COO- of anion (Ce6) to measure the acid responsive ability of SiO2-PCe6-IL. As shown in Figure 3E, F, G and H, the valence peak of the N+-O- and C-O- in SiO2-PCe6-IL was disappeared in the acidic solution. As shown in Figure 3J, more than 90% of the Ce6 was released in 10 s. Compared with the traditional acid-sensitive bond, this special ionic bond could significantly increase the release rate of photosensitizer. With the protonation of Ce6, the charge of SiO2-PCe6-IL was inverted and the zeta potentials were changed from -0.3±3.4, -0.6±3.2 mV to +13.8±4.8 and 22.9±3.1 mV, respectively (Figure 3I). Although Ce6-IL was equimolar in SiO2-PCe6-IL1 and SiO2-PCe6-IL2, the changes in charge were different. Compared with SiO2-PCe6-IL2, the charge variation of SiO2-PCe6-IL1 was relatively weaker. This may also be caused by the winding of PCe6-IL1 with SiO2 which led to some positive charge neutralization with SiO2. The lower positive charge weakened the interaction of SiO2-PIL1+ with PNAG and then affected the punching ability of SiO2-PCe6-IL1.
The binding ability of SiO2-PCe6-IL with biofilm
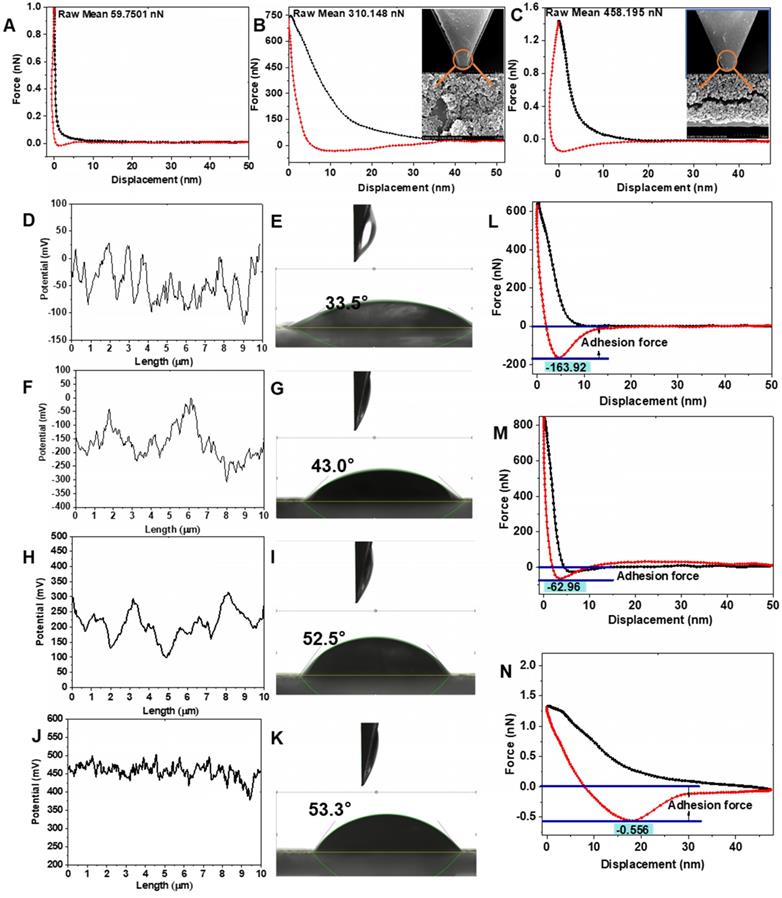
The SiO2-PIL1+ and SiO2-PIL2+ could bond with negatively charged EPS through electrostatic and hydrophobic interactions. As shown in Figure 4B and C, the interactions of SiO2-PCe6-IL1 and SiO2-PCe6-IL1 with EPS were 310.15 and 458.20 nN, respectively. However, the interaction of Ce6 with EPS was only 59.75 nN (Figure 4A). The high charged density polyionic liquids with short-chains greatly enhanced the interaction between SiO2-PCe6-IL2 and EPS. The stronger interaction provided an opportunity to combine and create holes in the biofilm.
Biofilm elimination in vitro
A semi-quantitative plate assay was used to test the concentration that could eliminate MRSA biofilm. After illumination for 15 min, the MRSA biofilm that was treated with Ce6 was not eliminated at 100 µM or even at 500 µM (Figure S8). Compared with the SiO2-PCe6-IL1, SiO2-PCe6-IL2 could eliminate MRSA biofilm at 100 µM. To further demonstrate the PDT efficiency of SiO2-PCe6-IL, the residual biofilms were dispersed under ultrasonication, and the bacterial viability was analyzed by plate counting. Figure S9 displays the visual images of the agar plates and summarizes the number of bacteria after treating with Ce6 and SiO2-PCe6-IL at 100 µM. The Ce6 alone could not destroy the MRSA bacteria embedded in the biofilm. With the “super-porogen”, the live stationary phase MRSA was significantly disrupted compared with the Ce6 group. Furthermore, the number of MRSA clearly decreased after treating with SiO2-PCe6-IL versus Ce6, particularly for SiO2-PCe6-IL2. This demonstrated that the SiO2-PCe6-IL with a short and high charged density chain not only effectively eradicated the biofilm but also inactivated the embedded MRSA.
The morphology and element analysis. (A) SEM of SiO2. (B) TEM of SiO2. (C) The Si and O analysis of SiO2. (D) SEM of SiO2-PCe6-IL1. (E) TEM of SiO2-PCe6-IL1. (F) The Si, O, C and N analysis of SiO2-PCe6-IL1. (G) SEM of SiO2-PCe6-IL2. (H) TEM of SiO2-PCe6-IL2. (I) The Si, O, C and N analysis of SiO2-PCe6-IL2. (J) The location analysis of N in SiO2-PCe6-IL1. (K) The location analysis of Si in SiO2-PCe6-IL1. (L) The location of Si and N in SiO2-PCe6-IL1. (M) The location analysis of N in SiO2-PCe6-IL2. (N) The location analysis of Si in SiO2-PCe6-IL2. (O) The location of Si and N in SiO2-PCe6-IL2.
(A) The 1O2 production of Ce6 alone in DMSO. (B) The 1O2 production of SiO2-PCe6-IL1 in DMSO. (C) The 1O2 production of SiO2-PCe6-IL2 in DMSO. (D) The chemical species of N in SiO2-PCe6-IL. (E) The chemical species of C in SiO2-PCe6-IL. (F) The chemical species of N in SiO2-PIL+ when released Ce6 at 24 h. (G) The chemical species of C in SiO2-PIL+ when released Ce6 at 24 h. (H) Ce6 loading rate of SiO2-PCe6-IL1 and SiO2-PCe6-IL2. (I) Zeta potential of SiO2-PCe6-IL1 and SiO2-PCe6-IL2 in different pH solution. (J) The release rate of Ce6 in pH 4.5 solution.
The physicochemical properties of biofilm
To study the effect of SiO2-PCe6-IL on the properties of biofilm, the surface potential, hydrophobicity, mechanical and adhesion properties which are key factors in maintaining the structure and protection function of biofilm are examined [40, 41]. After adding Ce6, the surface potential of MRSA biofilm decreased from -52 to -101 mV due to the COO- of Ce6 that existed on the biofilm surface (Figure 4F). For the treatment group of SiO2-PCe6-IL1 and SiO2-PCe6-IL2, the surface potential increased from -42 to 265 and 450 mV, respectively (Figure 4H and J). SiO2-PCe6-IL2 had a great influence on the surface potential of MRSA biofilm as a short and high charged density structure of the polyimidazole cation made PIL2+ repel each other, and it was fully integrated with the biofilm. In addition to the change in surface potential, the hydrophobicity of the MRSA biofilm also increased from the insertion of hydrophobic dodecyl. The contact angles of the MRSA biofilm treated by SiO2-PCe6-IL1 and SiO2-PCe6-IL2 changed from 33.5° to 52.5° and 53.3°, respectively (Figure 4I and K). The Young's modulus of the MRSA biofilm was 598.12 kpa. After treating with Ce6, SiO2-PCe6-IL1 and SiO2-PCe6-IL2, the Young's modulus of the biofilm was 435.29, 273.95, and 149.19 kpa, respectively (Figure S10). The mechanical properties after treating with SiO2-PCe6-IL2 degraded significantly. The weakened mechanical properties demonstrated that the stronger interaction between SiO2-PCe6-IL2 and MRSA biofilm could more effectively destroy the structural integrity of MRSA biofilm. In addition, as the mechanical stability was damaged, the adhesion force of the MRSA biofilm treated by SiO2-PCe6-IL1 or SiO2-PCe6-IL2 was also decreased from 163.9 nN to 62.96 and 0.556 nN, respectively (Figure 4M and N). Compared with SiO2-PCe6-IL1, SiO2-PCe6-IL2 almost completely eliminated the adhesion of the MRSA biofilm. This probably means that the short-chained and high-density poly-imidazole cations could efficiently combine with sticky substances, and then quickly eliminated the adhesion. These results demonstrated that SiO2-PCe6-IL2 with a short and high density poly-dodecyl-imidazole cations could more effectively damage the physical and chemical properties of MRSA biofilm.
The hole-making ability
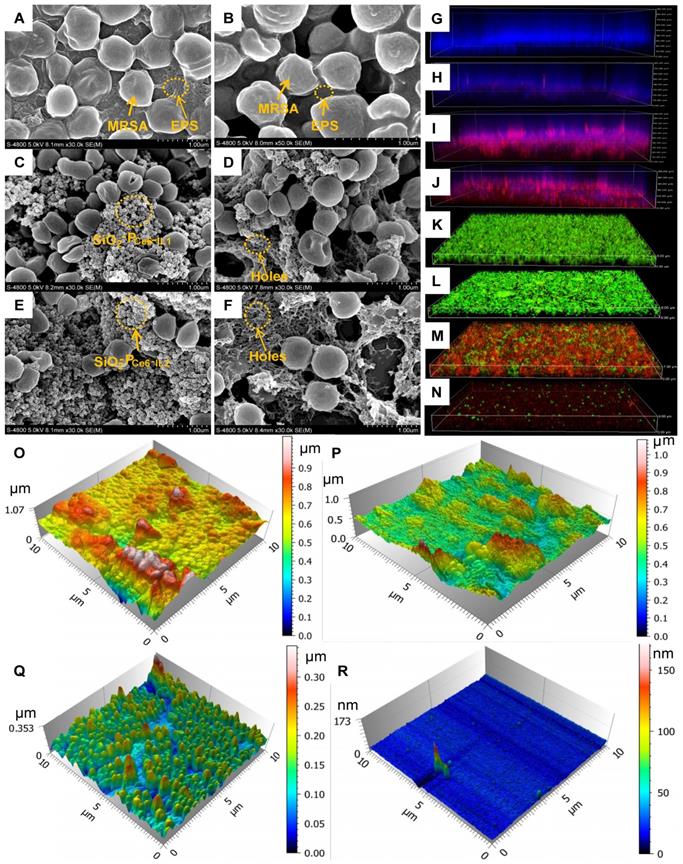
The surface potential, hydrophobicity, mechanical and adhesion force must be changed to destroy the structural integrity of the biofilm. As shown in Figure 5ⅰ, the MRSA biofilm composed EPS and incorporated MRSA bacteria. For the charge reversal, SiO2-PIL1+ and SiO2-PIL2+ could firmly adsorb on the EPS (Figure 5C and E). After interaction for 10 min, many holes in the EPS were actually observed by SEM (Figure 5D and F), especially for SiO2-PCe6-IL2.
The interaction between biofilm and Ce6 (A), SiO2-PCe6-IL1 (B), SiO2-PCe6-IL2 (C). (D) The surface potential of MRSA. (E) The contact angle of MRSA. (F) The surface potential of biofilm treated with Ce6. (G) The contact angle of biofilm treated with Ce6. (H) The surface potential of biofilm treated with SiO2-PCe6-IL1. (I) The contact angle of biofilm treated with SiO2-PCe6-IL1. (J) The surface potential of biofilm treated with SiO2-PCe6-IL2. (K) The contact angle of biofilm treated with SiO2-PCe6-IL2. The adhesion force of biofilm treated with Ce6 (L), SiO2-PCe6-IL1 (M), SiO2-PCe6-IL2 (N).
The morphology of MRSA biofilm. (A) MRSA biofilm. (B) Treated with Ce6 for 10 min. (C) Treated with SiO2-PCe6-IL1 for 10 min. (D) The holes of MRSA biofilm after SiO2-PCe6-IL1 removing. (E) Treated with SiO2-PCe6-IL2 for 10 min. (F) The holes of MRSA biofilm after SiO2-PCe6-IL2 removing. (G) MRSA biofilm (blue). (H) The fluorescence imaging (red) of Ce6 in biofilm after treated with Ce6 alone. (I) The fluorescence imaging of Ce6 in biofilm after treated with SiO2-PCe6-IL1. (J) The fluorescence imaging of Ce6 in biofilm after treated with SiO2-PCe6-IL2. (K) The live (green) & dead (red) bacteria in biofilm. (L) The live & dead bacteria in biofilm after treated by Ce6 with illumination for 15 min (5 mW/cm2). (M) The live &dead bacteria in biofilm after treated by SiO2-PCe6-IL1 with illumination for 15 min (5 mW/cm2). (N) The live&dead bacteria in biofilm after treated by SiO2-PCe6-IL2 with illumination for 15 min (5 mW/cm2). (O) The morphology of MRSA biofilm. (P) The morphology of MRSA biofilm treated by Ce6 with illumination for 15 min (5 mW/cm2). (Q) The morphology of MRSA biofilm treated by SiO2-PCe6-IL1 with illumination for 15 min (5 mW/cm2). (R) The morphology of MRSA biofilm treated by SiO2-PCe6-I2 with illumination for 15min (5 mW/cm2).
Location and ROS of Ce6 in the biofilm
The biofilms as a natural barrier prevent the photosensitizer from entering. After the SiO2-PCe6-IL treatment, Ce6 could easily enter into the biofilm through holes and concentrate in the biofilm. The location of Ce6 was confirmed by a CLSM. As shown in Figure 5G, the MRSA biofilm exhibited integrity (blue) and it was difficult for Ce6 alone enter (Figure 5H). However, the blue fluorescence intensity of the MRSA biofilm treated by SiO2-PCe6-IL1 and SiO2-PCe6-IL2 was decreased, and a high concentration of Ce6 was detected in the MRSA biofilm (Figure 5I and J). More importantly, the high concentration of ROS after treating with SiO2-PCe6-IL was observed in the biofilm (Figure S11). Because of the effective accumulation of ROS in the biofilm, almost all of the MRSA bacteria were killed by SiO2-PCe6-IL2 (Figure 5M and N).
The morphology of the MRSA biofilm
The MRSA biofilm treated with Ce6, SiO2-PCe6-IL1 or SiO2-PCe6-IL2 with illumination was examined by AFM and SEM. As shown in Figure 5B and P, the treatment of Ce6 alone had almost no effect on the MRSA embedded in biofilm other than a slight influence on the surface structure of the biofilm. However, the structure and morphology MRSA biofilm was destroyed after treated by SiO2-PCe6-IL1 or SiO2-PCe6-IL2 with illumination for 15 min, especially for SiO2-PCe6-IL2 (Figure 5Q, R and Figure S12).
Anti-biofilm activity in vivo
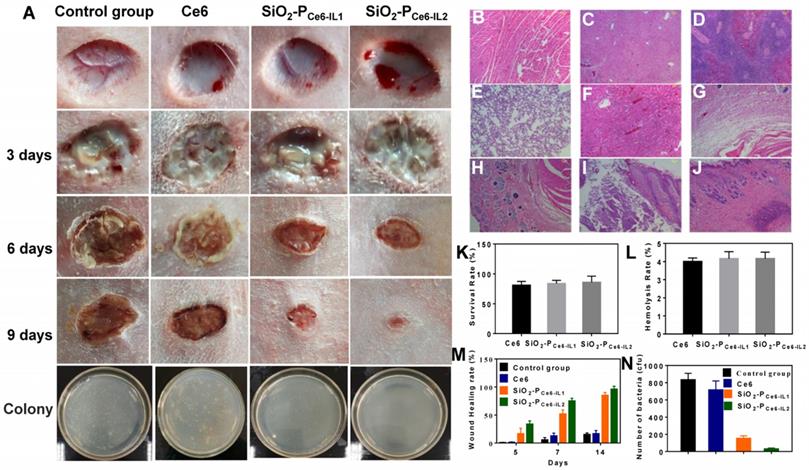
To assess the anti-biofilm activity in vivo, the cutting model was fabricated on the back of rabbit. The wounds were injected with 50 μL of 108 cfu/mL MRSA to construct the MRSA biofilm infection model. The infected rabbits were divided into four groups: treated by PBS, Ce6, SiO2-PCe6-IL1 and SiO2-PCe6-IL2 with illumination for 15 min. Figure 6 showed the photographs of the wounds in 1-9 days. All the infected groups showed certain degree of pyosis in 3 days. 50 µL of PBS, Ce6, SiO2-PCe6-IL (Ce6 100 µM) was dropped on the wound area at the corresponding groups and irradiated with 660 nm light (5 mW/cm2) for 15 min. In 6 days, the pyosis of infected wounds that treated with SiO2-PCe6-IL1 and SiO2-PCe6-IL2 disappeared. In 9 days, the group of SiO2-PCe6-IL2 showed better healing than other groups (Figure 6M). To assess the bactericidal effect on the wounds, the MRSA on the wounds at 14 days were cultured, and then colonies were counted. For only few colonies formed after incubating for 24 h (Figure 6N), the SiO2-PCe6-IL2 exhibited the remarkable therapeutic effect for combating MRSA biofilm infection.
Biocompatibility assay
As SiO2-PCe6-IL was in direct contact with tissues and blood in practical clinical applications, the biocompatibility was evaluated. The cytotoxicity and hemolysis rate of the SiO2-PCe6-IL1 and SiO2-PCe6-IL2 at 100 µM were above 90% and less than 5%, respectively (Figure 6K and L). In addition, to evaluate the safety of the SiO2-PCe6-IL2, the heart, liver, spleen, lung, kidney and embedded tissue were also harvested for H&E staining. The pathological and histopathological studies showed that SiO2-PCe6-IL could not cause the damage to embedded tissue and major organs. The SiO2-PCe6-IL could be as a safe material against biofilm infection.
Antibacterial effect in vivo and biocompatibility assay. (A) Photomicroscope images of wounds at different days. H&E staining. (B) Heart. (C) Liver. (D) Spleen. (E) Lung. (F) Kidney. (G) Subcutaneous. (H) Wound areas. (I) Infected skin tissue. (J) Infected skin treated with SiO2-PCe6-IL2. (K) Cytotoxicity. (L) Hemolysis rate. (M) Wound healing rate. (N) The number of bacterial colony-forming units obtained from control, and after treated by Ce6, SiO2-PCe6-IL1, SiO2-PCe6-IL2 with illumination for 15 min (50 µL of Ce6, SiO2-PCe6-IL and SiO2-PCe6-IL2 (containing 100 µM Ce6), 5 mW/cm2).
Discussion
In summary, we reported a novel antibacterial system to solve the release and transport barrier problem of photosensitizers. Compared with the traditional system, the SiO2-PCe6-IL could rapidly concentrate the photosensitizers in biofilm and control infection at the early stages. The in vitro and in vivo results indicated that SiO2-PCe6-IL can effectively reduce the inflammatory stage of the wound and accelerate wound healing. The biocompatibility results indicated that SiO2-PCe6-IL could be an effective and safe therapeutic method for controlling MRSA biofilm infection. Furthermore, the highly efficient utilization of photosensitizers could reduce economic losses. As Ce6 has an excellent bactericidal effect on Gram-positive bacteria, the SiO2-PCe6-IL is expected to be an effective strategy for other positive bacterial biofilm infections in clinical applications.
Materials and Methods
Materials
1, 3-diphenylisobenzofuran (DPBF) and 3-(4, 5-dimethyl thiazol-2-yl)-2, 5-diphenyl-tetrazolium bromide (MTT) were purchased from Sigma-Aldrich. Ce6 was purchased from Frontier Scientific. KOH, Triton x-100, methanol, absolute ethanol, cyclohexane, hexanol, and triethylamine were purchased from Sinopharm Chemical Reagent Co., Ltd. Crystal violet, N, N, N', N'', N''-pentamethyldiethylenetriamine, CuCl, 2-bromoisobutyryl bromide, tetraethyl orthosilicate (TEOS), ammonia solution, and 3-aminopropyl triethoxysilane (APTES) were purchased from Aladdin. 1-vinyl-3-dodecyl imidazole bromide (IL) was kindly provided by the Key Laboratory of Space Applications Physics and Chemistry, Northwestern Polytechnical University. Methicillin-resistant Staphylococcus aureus (MRSA ATCC 33591) was provided by Xijing Hospital (The resistant criterion of MRSA to the drugs as follows: the MIC of OX, AK and EM was 0.486, 0.489 and 0.491 mg/mL, respectively; the MIC of CL and CIP was 0.015 and 0.063 mg/mL, respectively). LB-medium and agar were purchased from MP Biomedicals. Twenty-four pore plate circular cell crawling slices were purchased from WHB (WHB-24-CS, China). Alexa Fluor 647 was purchased from Thermo Fisher Scientific. Cellular ROS Assay Kit (deep red) ab 186029 was purchased from abcam.
The interaction of cation and PNGA
The binding energy (ΔE) of 1-vinyl-3-dodecyl imidazole and poly 1-vinyl-3-dodecyl imidazole (1 unit) to PNGA was calculated using Materials Studio. The simulation parameters were as follows: Forcite (module), universal (forcefield), current (charge), fine (quality), atom-based (electrostatic), van der Waals, cubic spline (truncation) cutoff distance of 12.5 Å, spline of 1 Å, and buffer width of 0.5 Å.
Synthesis and characterization of 1-vinyl-3-dodecyl imidazole Ce6 (Ce6-IL)
The cation 1-vinyl-3-dodecyl imidazole bromide (IL) and anion Ce6 were assembled into Ce6-IL by an anion exchange reaction. The characterization of Ce6-IL was reported in our previous reports [35]. The pKa values of the carboxylic acid groups of Ce6-IL have been determined by titration with NaOH according to the references [42]. The pH of the solution was measured using a calibrated glass electrode on a pH meter (M-T FE28, Switzerland) at 25°C.
Preparation of SiO2-Br
SiO2 nanoparticles were synthetized by three phase emulsion polymerization. In briefly, 38.0 mL of cyclohexane, 12.0 mL of Triton x-100, 8.0 mL of hexanol and 2.0 mL of distilled water were added to a flask and stirred for 30 min at 1100 rpm; then 500 μL of TEOS and 1.8 mL of ammonia (25%) were added for a reaction for 24 h at room temperature. The obtained SiO2 was washed several times by distilled water, ethanol and dried by vacuum freeze-drying. To study the influence of chain length and charge density on antibacterial properties, Br of two different densities was grafted onto the surface of SiO2. First, 50 mg of SiO2 was dispersed in anhydrous ethanol, and then 60 µL of APTES was added for a reaction for 48 h at 70 ℃. After washing with alcohol and water three times, SiO2-NH2 was obtained. Second, the SiO2-NH2 dissolved into anhydrous acetonitrile and 0.2 mL of 2-bromoisobutyryl bromide, 0.4 mL of anhydrous three ethylamine or 1.0 mL of 2-bromoisobutyryl bromide, and 2.0 mL of anhydrous three ethylamine were added for a reaction for 12 h in an ice bath. After the reaction, the two Br of different densities was washed by ethanol. The percentage of Br was examined by EDX.
Preparation and characterization of SiO2-PCe6-IL
Poly Ce6-IL of different chain lengths and charge densities were grafted onto SiO2 by ATRP. In briefly, SiO2-Br of two different densities were dissolved in 85% ethanol solution. After ultrasonic dispersion, 50 mg of Ce6-IL, 200 μL of PMDTA and 30 mg of CuCl were added into a flask for a reaction for 6 h under nitrogen protection. The morphology of SiO2-PCe6-IL was examined by TEM and SEM. The zeta potential of SiO2-PIL1+ and SiO2-PIL2+ in solutions with a pH of 7.4 and 4.5 were measured at 25 °C by a Delsa Nano C particle analyzer (Beckman Coulter Ireland Inc.).
The loading and release rate of Ce6
1.0 mg of SiO2-PCe6-IL was dispersed in a solution with a pH of 7.4. The absorbency of Ce6 at 660 nm was examined by UV-Vis (MAPADA, China). The Ce6 loading rate was eventually calculated by loading Ce6/carrier weight×100%. After the two kinds of SiO2-PCe6-IL were dispersed in a solution with a pH of 4.5, centrifugate was collected at 5 and 10 s by ultrafiltration. Then, the absorbency of Ce6 in centrifugate was examined, and the release rate was calculated.
The generation of ROS and 1O2 assay
DPBF was used as a probe to measure the generation of 1O2 according to the literature [43]. DMSO solution (2.0 mL) containing SiO2-PCe6-IL (Ce6 100 μM) and DPBF (100 μM) was irradiated by 660 nm light (5 mW/cm2). The absorbance of DPBF at 410 nm was recorded when illuminated for 1 and 2 min. As the control group, the ultraviolet absorption of Ce6 and SiO2-PCe6-IL at 410 nm without DPBF was detected after illumination for 1 and 2 min. ROS generation in living cells could detect using Cellular ROS Assay or DCFH-DA [44, 45]. In this work, the ROS of Ce6 in the MRSA biofilm wan detected by Cellular ROS Assay (deep red). After the Ce6, SiO2-PCe6-IL1 and SiO2-PCe6-IL1 (Ce6 100 μM) solutions combined with MRSA biofilm 10 min, Cellular ROS Assay was added. The ROS was produced with the 660 nm laser irradiation. The fluorescence imaging of ROS was evaluated by confocal laser scanning microscopy (CLSM). The excitation and emission wavelengths were 650 and 675 nm, respectively.
Culturing MRSA biofilm
The cell crawling slices and 2.0 mL of MRSA (1×107 cfu/mL) in LB medium (2% sucrose) with a pH of 4.5 were placed into a 24 pore-plate and MRSA biofilm was cultured at 37 °C for 72 h. After the LB medium was removed, the MRSA biofilm attached on the slices was harvested. According to the literature [46], the characterization of MRSA was as follows: the adhesion was 163.92 nN, the thickness was 90 µM, and the contact angle of the surface was 33.5° (hydrophilic).
Biofilm elimination in vitro
The elimination of MRSA biofilm was examined by semi-quantitative determination with crystal violet staining [47]. First, the obtained MRSA biofilm was rinsed briefly by PBS to remove planktonic bacteria. Afterward, 20 μL of different concentrations of Ce6 and SiO2-PCe6-IL were added into 96-well plates to interact with the biofilm for 10 s, and then illuminated for 15 min (5 mW/cm2). Second, the residual biofilm was stained with 200 μL of 1.0% crystal violet solution for 30 min, and 200 μL of ethanol was added to dissolve the crystal violet. The concentrations of Ce6 and SiO2-PCe6-IL ranged from 0 to 500 μM (0, 0.01, 0.05, 0.1, 1.0, 50, 100 and 500 μM).
Photodynamic inactivation of biofilm
MRSA biofilm was cultured for 72 h in 96-well plates and washed with PBS three times. Then, 50 µL of PBS, Ce6 and two kinds of SiO2-PCe6-IL (Ce6 100 µM) were added into each well and allowed to interact with biofilm for 10 min. Afterward, the 96-well plates were subjected to 660 nm irradiation for 15 min (5 mW/cm2). To quantify the viable bacteria, the residual biofilm was detached via low-energy sonication to obtain bacterial suspensions in 1.0 mL of PBS. Then the serially diluted bacteria suspensions were plated on LB agar incubation at 37 °C for 24 h, and the colonies forming units were counted.
Interaction of SiO2-PCe6-IL with biofilm
The interactions between Ce6, SiO2-PCe6-IL and MRSA biofilm were measured by AFM. A common protocol was employed for the attachment of SiO2-PCe6-IL1, SiO2-PCe6-IL2 or Ce6 to the AFM tip: the AFM tip (NP-O10, Bruker) was placed in epoxy, which was allowed to cure for some time (total 5 min) [48], 1 µL of SiO2-PCe6-IL1, SiO2-PCe6-IL2 and Ce6 (120 µM) were placed on the AFM tip. After drying at 80 ℃ for 24 h, the decorated tip was washed by distilled water to eliminate unattached nanoparticles. SEM was used to examine the SiO2-PCe6-IL nanoparticles that terminated on the AFM tip.
The influence of SiO2-PCe6-IL on biofilm
The changes in the physicochemical properties of the MRSA biofilm were examined by AFM (Dimension FastScan and Dimension Icon, Bruker, Germany) and a contact angle measuring instrument (OCA200, Dataphysics, Germany). The detailed processes were as follows: MRSA biofilm was treated with PBS, 100 µM of Ce6 and SiO2-PCe6-IL. After interaction for 10 min, the surface potential, contact angle, mechanical and adhesion properties of MRSA biofilm were examined. The Young's modulus of the MRSA biofilm was examined by Nano Indenter (Piuma, Optics11, Holland).
Analysis of the hole-forming ability and morphology of the biofilm
To study the hole-forming ability, three pieces of MRSA biofilm treated with 100 µM of Ce6 and SiO2-PCe6-IL without illumination were examined. After interaction for 10 min, the three pieces of MRSA biofilm were washed several times with PBS to remove Ce6 and SiO2-PCe6-IL. The effect of superporogen and PDT on the morphology of MRAS biofilm was also examined using three pieces of MRSA biofilm treated with 100 µM of Ce6 and SiO2-PCe6-IL1 with illumination for 15 min. AFM and SEM were used to examine the changes in the biofilm.
Location of photosensitizer
The biofilm was stained and examined with confocal scanning laser microscopy (CSLM) [49]. In briefly, 1.0 µM of Alexa Fluor 647-labeled dextran conjugate (molecular weight 10,000; absorbance wavelength 647 nm; emission wavelength 668 nm) was added to culture for 6 h. Then, 20 µL of SiO2-PCe6-IL1, SiO2-PCe6-IL2 or Ce6 was added to the biofilm to interact for 10 min, after which the biofilm was washed with PBS 3 times to remove residual SiO2-PCe6-IL or Ce6. The CSLM imaging was performed using a Leica TCS SP1 microscope (Leica TCS SP8 STED 3X Super-resolution Confocal Microscope, Germany) equipped with argon ion and helium-neon lasers set at 400 and 640 nm, respectively.
Animal studies
Healthy New Zealand white rabbits were used in the animal study. The MRSA infected wounds were prepared on the backs of the rabbits. In briefly, 8 week-old rabbits (1.5-1.8 kg) were purchased from the Laboratory Animal Center of the Fourth Military Medical University and divided into four groups: PBS, Ce6, SiO2-PCe6-IL1 and SiO2-PCe6-IL2 (three rabbits in each group). The rabbits were anesthetized by 2% sodium pentobarbital. After shaving and disinfecting with alcohol, the wounds (d=2 cm) were obtained by surgical procedure on the backs of the rabbits. The infected wounds were treated by a 50 μL of 108 cfu/mL MRSA suspension. When a biofilm-infected wound was observed, the 50 µL or 100 µM of Ce6 or SiO2-PCe6-IL was dropped on the wound area for the corresponding groups. The wounds treated by PBS served as the control group. After 10 min, the infected wound area was irradiated for 15 min (660 nm, 5 mW/cm2). The wounds were photographed to observe the healing rate of the wound. To check the antibacterial activity in vivo, the bacterial samples treated with PBS, Ce6, SiO2-PCe6-IL1 or SiO2-PCe6-IL2 at 14 days were collected from the wound area by sterile swab. After culturing for 8 h and diluting 1,000 times, 10 μL of bacterial suspension was spread on the agar culture plate and incubated at 37 ℃ for 24 h to count the number of colonies.
Biocompatibility assay
The cell viability was evaluated by MTT assays. Normal L929 fibroblast cells were seeded into 96-well plates (6000 cells per well) with 200 μL of DMEM culturing medium in each well for 24 h. Ce6, SiO2-PCe6-IL1 or SiO2-PCe6-IL2 with concentrations of 100 µM were added to the cells and irradiated by 660 nm light for 15 min (5 mW/cm2). After incubation for 48 h, 20 μL of MTT solution (0.1 mg/mL) was added to each well for another 4 h culturing. Then, the medium was removed, and 150 µL of DMSO was added to each well to dissolve the obtained crystals. The absorbance was recorded at 570 nm by a microplate reader (model 550 BioRad).
Fresh blood (3.0 mL) was obtained from the New Zealand white rabbit. After the red cells were diluted to 2% in PBS, Ce6 or SiO2-PCe6-IL (100 µM) was immersed into a tube (5.0 mL for each tube) and incubated at 37 ℃ for 3 h. The red cells were centrifuged and the supernatant containing hemoglobin was detected using UV-Vis. The OD values were recorded at 540 nm. The red cells with water and PBS were the positive control and negative control, respectively. The hemolysis rate was determined by the following equation [50-52]:
The positive control and the negative control were water and normal saline, respectively.
SiO2-PCe6-IL2 was implanted subcutaneously for 4 weeks. After the experiment, the embedded tissue, wound tissue, heart, liver and spleen, lungs and kidneys were harvested to study the biocompatibility by H&E staining analysis.
All experimental animal operating procedures were in line with the laboratory animal care and usage guidelines.
All data were expressed as the means ± SD. Differences between groups were examined for significance with a two-tailed Student's test and significance was set at p < 0.05.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 81571786, 31771087, 31671015, 81702246 and 31570997), Shaanxi Natural Science (No. 2018JM2031), and Shaanxi Key Research & Development Program Foundation (No. 2019SF-069).
Supplementary Material
Supplementary figures.
Author Contributions
Chaoli Wang, Peng Chen, and Youbei Qiao designed the experiments. Yuan Kang and Chaoren Yan performed the experiments. Zhe Yu and Xin He analyzed the data and prepared figures. Jian Wang provided technical support. Hong Wu wrote the manuscript.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Liu Y, Shi LQ, Su LZ, van der Mei HC, Jutte PC, Rene YJ. et al. Nanotechnology-based Antimicrobials and Delivery Systems for Biofilm-Infection. Chem Soc Rev. 2019;48:428-446
2. Chen JJ, Shi XT, Zhu Y, Chen YH, Gao M, Gao HC. et al. On-Demand Storage and Release of Antimicrobial Peptides using Pandor's Box-Like Nanotubes Gated with a Bacterial Infection-Responsive Polymer. Theranostics. 2020;10:109-122
3. Chen XK, Zhang XD, Lin FM, Guo YX, Wu FG. One-Step Synthesis of Epoxy Group-Terminated Organosilica Nanodots: A Versatile Nanoplatform for Imaging and Eliminating Multidrug-Resistant Bacteria and Their Biofilms. Small. 2019;15:1901647
4. Landis RF, Li CH, Gupta A, Lee YW, Yazdani M, Ngernyuang N. et al. Biodegradable Nanocomposite Antimicrobials for the Eradication of Multidrug-Resistant Bacterial Biofilms without Accumulated Resistance. J Am Chem Soc. 2018;140:6176-6182
5. Li JH, Zhang KX, Ruan L, Chin SF, Wickramasinghe N, Liu HB. et al. Block Copolymer Nanoparticles Remove Biofilms of Drug-Resistant Gram-Positive Bacteria by Nanoscale Bacterial Debridement. Nano Lett. 2018;18:4180-4187
6. He XW, Xiong LH, Zhao Z, Wang ZY, Luo L, Lam JWY. et al. AIE-based Theranostic Systems for Detection and Killing of Pathogens. Theranostics. 2019;9:3223-3248
7. Ran HH, Cheng XT, Bao YW, Hua XW, Gao G, Zhang XD. et al. Multifunctional Quaternized Carbon Dots with Enhanced Biofilm Penetration and Eradication Efficiencies. J Mater Chem B. 2019;7:5104-5114
8. Foote CS. Definition of Type I and Type II Photosensitized Oxidation. Photochem Photobiol. 1991;54:659-659
9. Cai Q, Fei Y, An HW, Zhao XX, Ma Y, Cong Y. et al. Macrophage-Iinstructed Intracellular Staphylococcus aureus Killing by Targeting Photodynamic Dimers. ACS Appl Mater Interfaces. 2018;10:9197-9202
10. Owusu EGA, MacRobert AJ, Naasani I, Parkin IP, Allan E, Yaghini E. Photoactivable Polymers Embedded with Cadmium-Free Quantum Dots and Crystal Violet: Efficient Bactericidal Activity against Clinical Strains of Antibiotic-Resistant Bacteria. ACS Appl Mater Interfaces. 2019;11:12367-12378
11. Hynek J, Zelenka J, Rathouský J, Kubát P, Ruml T, Demel J. et al. Designing Porphyrinic Covalent Organic Frameworks for the Photodynamic Inactivation of Bacteria. ACS Appl Mater Interfaces. 2018;10:8527-8535
12. Chen XK, Zhang XD, Lin FM, Gu YX, Wu FG. One-Step Synthesis of Epoxy Group-Terminated Organosilica Nanodots: A Versatile Nanoplatform for Imaging and Eliminating Multidrug-Resistant Bacteria and Their Biofilms. Small. 2019;15:1901647
13. Zhai Y, Busscher HJ, Liu Y, Zhang ZK, Kooten TGV, Su LZ. et al. Photoswitchable Micelles for the Control of Singlet-Oxygen Generation in Photodynamic Therapies. Biomacromolecules. 2018;19:2023-2033
14. Pushalkar S, Ghosh G, Xu QF, Liu Y, Ghogare AA, Atem C. et al. Superhydrophobic Photosensitizers: Airborne 1O2 Killing of an in Vitro Oral Biofilm at the Plastron Interface. ACS Appl Mater Interfaces. 2018;10:25819-25829
15. Fanesi A, Paule A, Bernard O, Briandet R, Lopes F. The Architecture of Monospecific Microalgae Biofilms. Microorganisms. 2019;7:352
16. Jia RN, Tian WG, Bai HT, Zhang JM, Wang S, Zhang J. Sunlight-Driven Wearable and Robust Antibacterial Coatings with Water-Soluble Cellulose-Based Photosensitizers. Adv Healthc Mater. 2019;8:1801591
17. Dai XM, Zhao Y, Yu YJ, Chen XL, Wei XS, Zhang XG. et al. All-in-one NIR-Activated Nanoplatforms for Enhanced Bacterial Biofilm Eradication. Nanoscale. 2018;10:18520-18530
18. Chen LH, Bai HT, Xu JF, Wang S, Zhang X. Supramolecular Porphyrin Photosensitizers: Controllable Disguise and Photoinduced Activation of Antibacterial Behavior. ACS Appl Mater Interfaces. 2017;9:13950-13957
19. Hu DF, Li H, Wang BL, Ye Z, Lei WX, Jia F. et al. Surface-Adaptive Gold Nanoparticles with Effective Adherence and Enhanced Photothermal Ablation of Methicillin-Resistant Staphylococcus aureus Biofilm. ACS Nano. 2017;11:9330-9339
20. Wei T, Yu Q, Chen H. Responsive and Synergistic Antibacterial Coatings: Fighting Against Bacteria in a Smart and Effective Way. Adv Healthc Mater. 2019;8:1801381
21. Huang JJ, Ren JN, Chen GP, Li ZG, Liu Y, Wang GF. et al. Tunable Sequential Drug Delivery System based on Chitosan/Hyaluronic Acid Hydrogels and PLGA Microspheres for Management of Non-Healing Infected Wounds. Mater Sci Eng C. 2018;89:213-222
22. Elder K, Baker DJ, Ribes JA. Infections, Infertility, and Assisted Reproduction. Cambridge, UK: Cambridge University Press. 2004
23. Gomes JM, Silva SS, Reis RL. Biocompatible Ionic Liquids: Fundamental Behaviours and Applications. Chem Soc Rev. 2019;48:4317-4335
24. Zaitsau DH, Emel'yanenko VN, Stange P, Verevkin SP, Ludwig R. Dissecting the Vaporization Enthalpies of Ionic Liquids by Exclusively Experimental Methods: Coulomb Interaction, Hydrogen Bonding, and Dispersion Forces. Angew Chem Int Ed Engl. 2019;58:8589-8592
25. Ries LAS, do Amaral FA, Matos K, Martini EMA, de Souza MO, de Souza RF. Evidence of Change in the Molecular Organization of 1-N-butyl-3-Methylimidazolium Tetrafluoroborate Ionic Liquid Solutions with the Addition of Water. Polyhedron. 2008;27:3287-3293
26. Thawarkar S, Khupse ND, Shinde DR, Kumar A. Understanding the Behavior of Mixtures of Protic-Aprotic and Protic-Protic Ionic Liquids: Conductivity, Viscosity, Diffusion Coefficient and Ionicity. J Mol Liq. 2019;276:986-994
27. Zajac A, Kukawka R, Pawlowska-Zygarowicz A, Stolarska O, Smiglak M. Ionic Liquids as Bioactive Chemical Tools for Use in Agriculture and the Preservation of Agricultural Products. Green Chem. 2018;20:4764-4789
28. Niemczak M, Kaczmarek DK, Klejdysz T, Gwiazdowska D, Marchska K, Pernak J. Ionic Liquids Derived from Vitamin C as Multifunctional Active Ingredients for Sustainable Stored-Product Management. ACS Sustain Chem Eng. 2019;71:1072-1084
29. Zhang YF, Zhou ZL, Zou L, Chi R. Imidazolium-Based Ionic Liquids with Inorganic Anions in the Extraction of Salidroside and Tyrosol from Rhodiola: The Role of Cations and Anions on the Extraction Mechanism. J Mol Liq. 2019;275:136-145
30. Hwang G, Koltisko B, Jin X, Koo H. Nonleachable Imidazolium-Incorporated Composite for Disruption of Bacterial Clustering, Exopolysaccharide-Matrix Assembly, and Enhanced Biofilm Removal. ACS Appl Mater Interfaces. 2017;9:38270-38280
31. Sun W, Wang YB, Zhang WX, Ying HJ, Wang P. Novel Surfactant Peptide for Removal of Biofilms. Colloids Surf B Biointerfaces. 2018;172:180-186
32. Eze EC, Chenia HY, Zowalaty MEE. Acinetobacter Baumannii Biofilms: Effects of Physicochemical Factors, Virulence, Antibiotic Resistance Determinants, Gene Regulation, and Future Antimicrobial Treatments. Infect Drug Resist. 2018;11:2277-2299
33. Duan F, Feng XC, Jin Y, Liu DW, Yang XJ, Zhou GQ. et al. Metalecarbenicillin Framework-based Nanoantibiotics with Enhanced Penetration and Highly Efficient Inhibition of MRSA. Biomaterials. 2017;144:155-165
34. Kruspe S, Meyer C, Hahn U. Chlorin e6 Conjugated Interleukin-6 Receptor Aptamers Selectively Kill Target Cells Upon Irradiation. Mol Ther Nucleic Acids. 2014;3:e143
35. Wang CL, Chen P, Qiao YB, Kang Y, Guo SY, Wu DF. et al. Bacteria Activated Chlorin e6 Ionic Liquid based on Cation and Anion Dual-Mode Antibacterial Action for Enhanced Antibacterial Efficacy. Biomater Sci. 2019;7:1399-1410
36. Liu Y, Busscher HJ, Zhao BR, Li YF, Zhang ZK, van der Mei HC. et al. Surface-Adaptive, Antimicrobially Loaded, Micellar Nanocarriers with Enhanced Penetration and Killing Efficiency in Staphylococcal Biofilms. ACS Nano. 2016;10:4779-4789
37. Egorova KS, Gordeev EG, Ananikov VP. Biological Activity of Ionic Liquids and Their Application in Pharmaceutics and Medicine. Chem Rev. 2017;117:7132-7189
38. Lin J, Chen XY, Chen CY, Hu JT, Zhou CL, Cai XF. et al. Durably Antibacterial and Bacterially Antiadhesive Cotton Fabrics Coated by Cationic Fluorinated Polymers. ACS Appl Mater Interfaces. 2018;10:6124-6136
39. Doumon NY, Houard FV, Dong JJ, Christodoulis P, Dryzhov MV, Portale G. et al. Improved Photostability in Ternary Blend Organic Solar Cells: the Role of [70] PCBM. J Mater Chem B. 2019;7:5104-5111
40. Liu XW, Xiao Y, Peng WG, Zhao LJ, Shen YM, Zhang SB. et al. New Designed DNA Light Switch Ruthenium Complexes as DNA Photocleavers and Topoisomerase I Inhibitors. Appl Organomet Chem. 2018;32:e4231
41. Abdel-Nour M, Su H, Duncan C, Li S, Raju D, Valton FSM. et al. Polymorphisms of a Collagen-like Adhesin contributes to legionella Pneumophila Adhesion, Biofilm Formation Capacity and Clinical Prevalence. Front Microbiol. 2019;10:604
42. Hajipour AR, Seddighi M. Pyridinium-Based Bronsted Acidic Ionic Liquid as Highly Efficient Catalyst for One-Pot Synthesis of Dihydropyrimidinones. Synth Commun. 2012;42:227-235
43. Allen A, Habimana O, Casey E. The Effects of Extrinsic Factors on the Structural and Mechanical Properties of Pseudomonas Fluorescens Biofilms: a Combined Study of Nutrient Concentrations and Shear Conditions. Colloids Surf B Biointerfaces. 2018;165:127-134
44. Dai XM, Zhao Y, Yu YJ, Chen X L, Wei XS, Zhang XG. et al. All-in-One NIR-Activated Nanoplatforms for Enhanced Bacterial Biofilm Eradication. Nanoscale. 2018;10:18520-18530
45. Chen H, Yang J, Sun L, Zhang HR, Guo YS, Qu J. et al. Synergistic Chemotherapy and Photodynamic Therapy of Endophthalmitis Mediated by Zeolitic Imidazolate Framework-Based Drug Delivery Systems. Small. 2019;15:1903880
46. Gowrishankar S, Kamaladevi A, Balamurugan K, Pandian SK. In Vitro and In Vivo Biofilm Characterization of Methicillin-Resistant Staphylococcus aureus from Patients Associated with Pharyngitis Infection. BioMed Res Int. 2016. 2016
47. Zhang K, Meng XD, Cao Y, Yang Z, Dong HF, Zhang YD. et al. Metal-Organic Framework Nanoshuttle for Synergistic Photodynamic and Low-Temperature Photothermal Therapy. Adv Funct Mater. 2018;28:1804634
48. Anderson MJ, Schaaf E, Breshears LM, Wallis HW, Johnson JR, Tkaczyk C. et al. Alpha-Toxin Contributes to Biofilm Formation among Staphylococcus aureus Wound Isolates. Toxins. 2018;10:157
49. Yang Y, Mao M, Lei L, Li M, Yin JX, Ma XX. et al. Regulation of Water-Soluble Glucan Synthesis by the Streptococcus mutans DexA Gene Effects Biofilm Aggregation and Cariogenic Pathogenicity. Mol Oral Microbiol. 2019;34:51-63
50. Zhu YN, Zhang JM, Song JY, Yang J, Du Z. Zhao WQ, et al. A Multifunctional Pro-Healing Zwitterionic Hydrogel for Simultaneous Optical Monitoring of pH and Glucose in Diabetic Wound Treatment. Adv Funct Mater. 2019. 1905 493
51. Zhang XY, Zhao YQ, Zhang YD, Wang AZ, Ding XK, Li Y. et al. Antimicrobial Peptide-Conjugated Hierarchical Antifouling Polymer Brushes for Functionalized Catheter Surfaces. Biomacromolecules. 2019;20:4171-4179
52. Yang X, Liu W, Xi GH, Wang MS, Liang B, Shi YF. et al. Fabricating Antimicrobial Peptide-Immobilized Starch Sponges for Hemorrhage Control and Antibacterial Treatment. Carbohydr Polym. 2019;222:115012
Author contact
![]() Corresponding author: Hong Wu (wuhongedu.cn). Department of pharmacy, Air Force Medical University, Xi'an, 710032, Shaanxi Province, China
Corresponding author: Hong Wu (wuhongedu.cn). Department of pharmacy, Air Force Medical University, Xi'an, 710032, Shaanxi Province, China
 Global reach, higher impact
Global reach, higher impact