13.3
Impact Factor
Theranostics 2020; 10(10):4659-4675. doi:10.7150/thno.42818 This issue Cite
Research Paper
Tumor reoxygenation for enhanced combination of radiation therapy and microwave thermal therapy using oxygen generation in situ by CuO nanosuperparticles under microwave irradiation
1. Laboratory of Controllable Preparation and Application of Nanomaterials, Key Laboratory of Cryogenics, Technical Institute of Physics and Chemistry, Chinese Academy of Sciences, No. 29 East Road Zhongguancun, Beijing 100190, People's Republic of China.
2. Department of Nuclear Medicine, Harbin Medical University Cancer Hospital, Harbin 150086, People's Republic of China.
3. Department of Interventional Ultrasound, Chinese PLA General Hospital, Beijing 100853, People's Republic of China.
4. Beijing Institute of Fashion Technology. No. A2, East Yinghua Street, Chaoyang District, Beijing 102209, People's Republic of China.
5. University of Chinese Academy of Sciences, Beijing 100049, People's Republic of China.
6. School of Information Engineering, Inner Mongolia University of Science and Technology, Baotou 014010, People's Republic of China.
Abstract
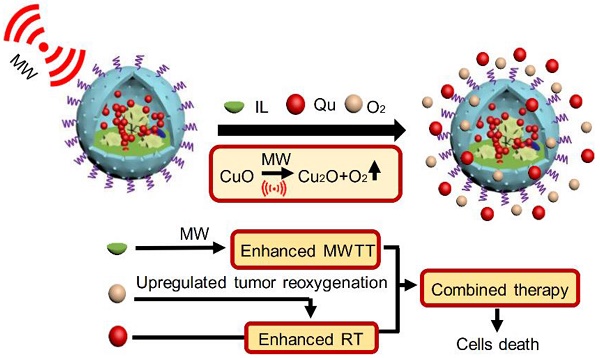
As known, radiation therapy (RT) can exacerbate the degree of hypoxia of tumor cells, which induces serious resistance to RT and in turn, is the greatest obstacle to RT. Reoxygenation can restore the hypoxic state of tumor cells, which plays an important role in reshaping tumor microenviroment for achieving optimal therapeutic efficacy. Herein, we report for the first time that microwave (MW)-triggered IL-Quercetin-CuO-SiO2@ZrO2-PEG nanosuperparticles (IQuCS@Zr-PEG NSPs) have been used to achieve an optimal RT therapeutic outcomes by the strategy of upregulating tumor reoxygenation, i.e. hypoxic cells acquire oxygen and return to normal state.
Methods: We prepared a promising multifunctional nanosuperparticle to upregulate tumor reoxygenation by utilizing CuO nanoparticle to generate oxygen under MW irradiation in the tumor microenvironment. The IQuCS@Zr-PEG NSPs were obtained by introducing CuO nanoparticles, MW sensitizer of 1-butyl-3-methylimidazolium hexafluorophosphate (IL), radiosensitizer of Quercetin (Qu) and surface modifier of monomethoxy polyethylene glycol sulfhyl (mPEG-SH, 5k Da) into mesoporous sandwich SiO2@ZrO2 nanosuperparticles (SiO2@ZrO2 NSPs). The release oxygen by IQuCS@Zr-PEG NSPs under MW irradiation was investigated by a microcomputer dissolved oxygen-biochemical oxygen demand detector (DO-BOD) test. Finally, we used the 99mTc-HL91 labeled reoxygenation imaging, Cellular immunofluorescence, immunohistochemistry, and TUNEL experiments to verify that this unique MW-responsive reoxygenation enhancer can be used to stimulate reshaping of the tumor microenvironment.
Results: Through experiments we found that the IQuCS@Zr-PEG NSPs can persistently release oxygen under the MW irradiation, which upregulates tumor reoxygenation and improve the combined tumor treatment effect of RT and microwave thermal therapy (MWTT). Cellular immunofluorescence and immunohistochemistry experiments demonstrated that the IQuCS@Zr-PEG NSPs can downregulate the expression of hypoxia-inducible factor 1α (HIF-1α) under MW irradiation. The 99mTc-HL91 labeled reoxygenation imaging experiment also showed that the oxygen generated by IQuCS@Zr-PEG NSPs under MW irradiation can significantly increase the reoxygenation capacity of tumor cells, thus reshaping the tumor microenvironment. The high inhibition rate of 98.62% was achieved in the antitumor experiments in vivo. In addition, the IQuCS@Zr-PEG NSPs also had good computed tomography (CT) imaging effects, which can be used to monitor the treatment of tumors in real-time.
Conclusions: The proof-of-concept strategy of upregulating tumor reoxygenation is achieved by MW triggered IQuCS@Zr-PEG NSPs, which has exhibited optimal therapeutic outcomes of combination of RT and MWTT tumor. Such unique MW-responsive reoxygenation enhancer may stimulate the research of reshaping tumor microenvironment for enhancing versatile tumor treatment.
Keywords: reoxygenation, tumor, microwave, thermal therapy, radiation therapy
 Global reach, higher impact
Global reach, higher impact