13.3
Impact Factor
Theranostics 2020; 10(10):4481-4489. doi:10.7150/thno.41646 This issue Cite
Research Paper
Human CD8 T cells are susceptible to TNF-mediated activation-induced cell death
1. Program of Immunology and Immunotherapy, Cima Universidad de Navarra, Pamplona, Spain.
2. Navarra Institute for Health Research (IDISNA), Pamplona, Spain.
3. Centro de Investigación Biomédica en Red de Cáncer (CIBERONC), Madrid, Spain.
4. Department of Pathology, Clínica Universidad de Navarra, Pamplona, Spain.
5. Department of Histology and Pathology, Universidad de Navarra, Pamplona, Spain.
6. Department of Oncology, Clínica Universidad de Navarra, Pamplona, Spain.
7. Department of Immunology and Immunotherapy, Clínica Universidad de Navarra, Pamplona, Spain.
*Senior co-authors.
Received 2019-10-30; Accepted 2020-2-21; Published 2020-3-15
Abstract
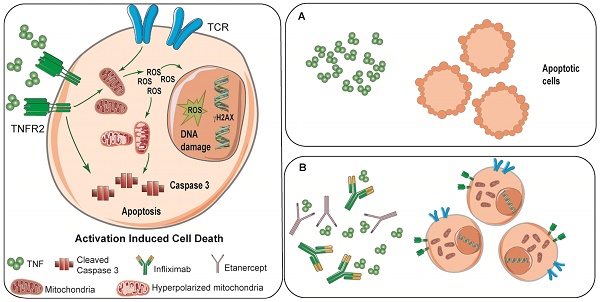
Activation-induced cell death (AICD) is a complex immunoregulatory mechanism that causes the demise of a fraction of T-lymphocytes upon antigen-driven activation. In the present study we investigated the direct role of TNF in AICD of CD8 T lymphocytes.
Methods: Human peripheral mononuclear cells were isolated from healthy donors and fresh tumor-infiltrating lymphocytes were obtained from cancer patients undergoing surgery. T cells were activated with anti-CD3/CD28 mAbs or with a pool of virus peptides, in combination with clinical-grade TNF blocking agents.
Results: A portion of CD8 T cells undergoes apoptosis upon CD3/CD28 activation in a manner that is partially prevented by the clinically used anti-TNF agents infliximab and etanercept. TNF-mediated AICD was also observed upon activation of virus-specific CD8 T cells and tumor-infiltrating CD8 T lymphocytes. The mechanism of TNF-driven T cell death involves TNFR2 and production of mitochondrial oxygen free radicals which damage DNA.
Conclusion: The use of TNF blocking agents reduces oxidative stress, hyperpolarization of mitochondria, and the generation of DNA damage in CD8 T celss undergoing activation. The fact that TNF mediates AICD in human tumor-reactive CD8 T cells suggests that the use of TNF-blocking agents can be exploited in immunotherapy strategies.
Keywords: TNF, AICD, mitochondria hyperpolarization, DNA damage
Introduction
Activation-induced cell death (AICD) is a regulated apoptotic process probably involved in the control of the intensity of T cell-mediated immune responses thereby avoiding excessive clonal expansions [1-3]. It is not only an essential mechanism to promote central immune tolerance inducing apoptosis for negative selection during thymocyte development [4], but it has also been proposed to play an important role in peripheral tolerance to self-antigens [5,6]. In mature T cells, a strong and chronic activation via T cell receptors may result in T cell hyperactivation leading to AICD [7,8]. This form of programmed cell death is mediated by surface receptors with an intracellular death domain and is executed by caspases. Among AICD-inducing receptors, FasL/CD95 is known to play an important role in T cell homeostasis and in the maintenance of immunological tolerance [9-11]. FasL is upregulated on activated T cells and can mediate activation-induced cell death (AICD), which physiologically eliminates activated T cells presumed to be no longer needed, with important implications for health and disease including autoimmunity and cancer [6,12-14].
Following activation, multiple T lymphocyte subsets produce TNF and lymphotoxin β. T cells express two types of lymphocyte receptors mainly TNFR2 and weakly TNFR1 [15]. TNFR1 encompasses a cytoplasmic death domain that recruits the adaptor proteins TNFR1-associated domain protein (TRADD) and Fas-associated death domain (FADD) thereby triggering the activation of the caspase cascade [16]. In contrast, TNFR2 recruits TNFR-associated factor 1 (TRAF1) and TRAF2, leading to NF-κB activation controlling the transcription of multiple genes [17]. Conceptually, TNF mostly relies on TNFR1 for apoptosis and on TNFR2 for any other function related to T cell function and survival. However, late in the T cell activation process, TNF induces a TNFR2-dependent pathway of cell death in CD8 T cells undergoing activation [18-20]. Studies both in TNFR1-/- and TNFR2-/- mice have shown roles for both TNFR1 and TNFR2 in AICD of murine T lymphocytes [18,21]. The mechanism of TNFR2-mediated cell death, however, remains elusive. Reportedly, TNF binding to TNFR2 results in apoptosis via RIP-mediated recruitment of FADD [22] or via depletion of TRAF2 and/or cIAPs [23]. Although TNFR1 is expressed by almost every mammalian cell type, TNFR2 expression is essentially restricted to immune cells and endothelial cells [15]. The consequence of these complex signaling routes in T lymphocytes is that TNF plays a dual role by acting as both an attenuator and mediator of immune responses.
In line with this, two recent reports have shown that blocking TNF in tumor-bearing mice enhances to some extent the antitumor effect of immunotherapy with anti-PD1 mAb or an anti-PD1 + anti-CTLA-4 combinations of mAbs, while at the same time TNF-blockade prevents associated immunopathology [24,25]. The effects promoting antitumor immunity could be traced to inhibition of AICD in tumor-reactive CD8 T lymphocytes [24].
We previously published that the apoptotic CD8 T cell death following PBMCs stimulation with anti-CD3/CD28 mAbs was partially inhibited with two neutralizing TNF agents, the TNF-trap etanercept and the monoclonal antibody infliximab [24]. In the current study, we sought to elucidate the mechanisms behind this phenomenon in human CD8 T cells and whether they are applicable to human T cells involved in antiviral and antitumor immunity.
Results
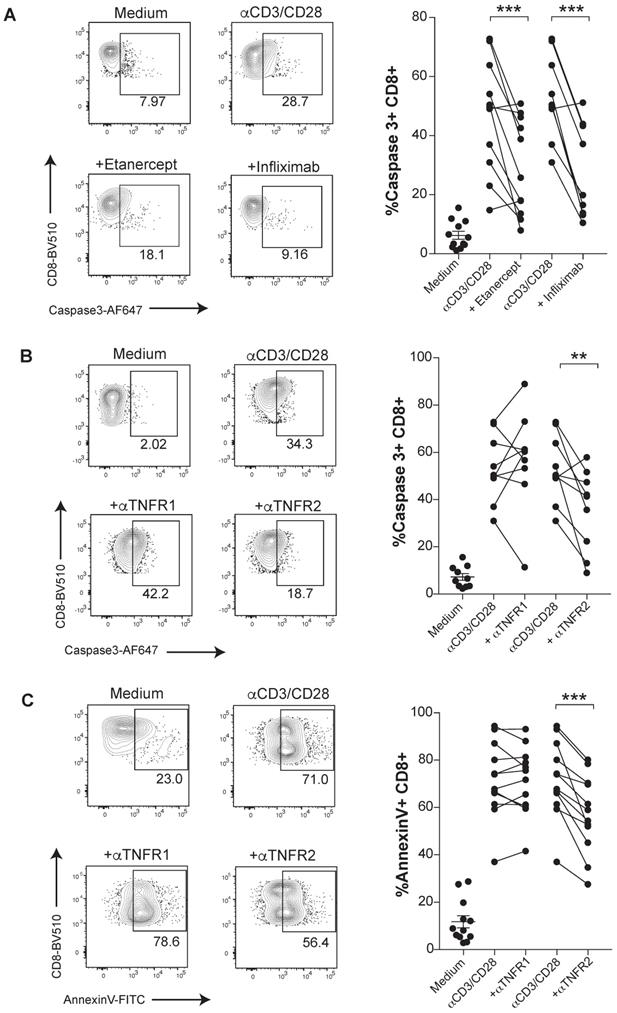
The susceptibility of activated human CD8 T cells to TNF-induced cell death triggered by anti-CD3/CD28 mAbs stimulation was confirmed in terms of a stronger proteolytic activation of caspase 3 (Figure 1A). Caspase 3 activation was mitigated under TNF blockade with etanercept and infliximab (Figure 1A). To determine which receptor was involved in the TNF-mediated AICD, we used specific blocking antibodies against either TNFR1 or TNFR2. We found a significant reduction of activated caspase 3 and surface Annexin V levels when CD8 T cells were treated with anti-TNFR2 mAb but not upon incubation with anti-TNFR1 mAb. Thus, in this cell-culture experimental system, TNF-mediated AICD is mainly dependent on TNFR2 (Figure 1B-C). In contrast, an inhibition of apoptotic activity by the anti-TNFR1 mAb on TNF-susceptible L929 cell line was documented (data not shown).
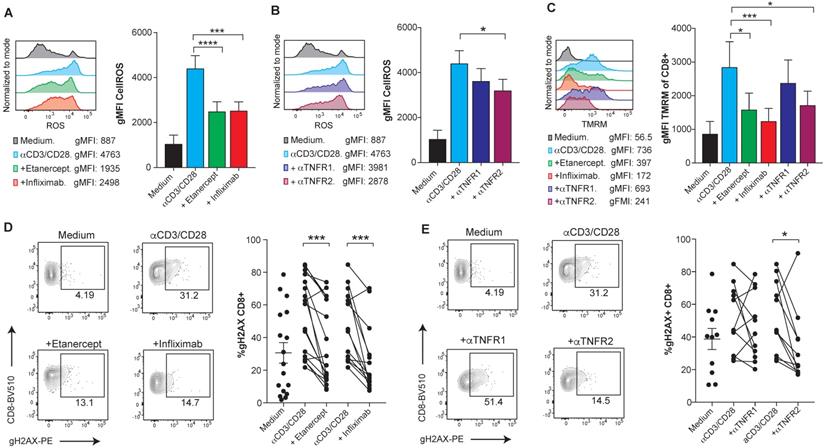
Reportedly, the mechanism that leads to TNF-induced cell death is at least in part mediated by the accumulation of intracellular reactive oxygen species (ROS), mainly produced by the mitochondria [26,27]. Using flow cytometry analysis, we observed elevated levels of intracellular ROS upon anti-CD3/CD28 activation of human CD8 T cells that were reduced upon TNF blockade and clearly decreased by anti-TNFR2 blocking mAb (Figure 2A-B). Moreover, blockade of TNF reduced the levels of mitochondrial transmembrane potential (ΔΨm) in CD8 T cells, as measured by TMRM, a lipophilic cationic dye that accumulates in the mitochondrial matrix in proportion to the magnitude of ΔΨm electronegativity [28] (Figure 2C). Enhancement of mitochondrial features, including transmembrane potential, is also observed in the case of another TNFR family member, 41BB (TNFR9) [29]. Attenuation of mitochondrial polarization by TNF blockade explains reduced ROS production as a byproduct of the electron transport chain. The assessment of TNF-induced DNA damage was measured by the detection of phosphorylated Histone H2AX (gH2AX). Flow-cytometry-assessed levels of gH2AX in CD8 T cells showed that TNF blockade decreases DNA damage in activated human CD8 T cells in a TNFR2 dependent manner (Figure 2D-E). Accordingly, oxidative stress and the DNA damage response, mechanistically link T-cell activation to apoptotic death.
In contrast, T cell effector functions were not affected upon TNFR1 or TNFR2 blockade, shown as the expression levels of CD25, PD-1, Ki67, Granzyme B and IFN-γ production of CD8 T cells after stimulation with anti-CD3/CD28 mAbs (Figure S1 A-E). Moreover, redirected cytolytic activity of CD8 T cells against a tumor cell line in the presence of anti-CD3-Epcam BiTE was not compromised by either TNFR1 or TNFR2 blockade (Figure S1F).
TNF induces apoptosis through TNFR2 in primary human CD8 T cells undergoing activation. PBMCs from healthy donors were activated with plate-bound anti-CD3 and soluble anti-CD28 mAbs for seven days in the presence or absence of blocking TNF agents. Apoptosis was measured by active caspase 3 staining of FACs-gated CD8 T cells, in the presence or absence of etanercept (n= 12) or infliximab (n= 10) (A), or selective blocking antibodies against TNFR1 or TNFR2 (n=12) (B). C, Surface Annexin V binding was evaluated on FACs-gated CD8 T cells in the indicated culture conditions testing TNFR1 or TNFR2 blockade (n=12). CD8 T cells were pre-gated on Live+/CD3+/CD4-. P values were calculated using a paired two-tailed t-test.
TNFR2 stimulation on human CD8 T cells increases ROS production via mitochondrial hyperpolarization and causes DNA damage. PBMCs from healthy donors were activated with plate-bound anti-CD3 and soluble anti-CD28 mAbs for seven days in the presence or absence of blocking TNF agents. Flow cytometry assessment of intracellular ROS geometric mean fluorescence in FACs-gated CD8 T cells stimulated in the presence or absence of etanercept or infliximab (A), or selective blocking antibodies against TNFR1 or TNFR2 (B) (n=8). C, Flow cytometry measurement of TMRM geometric mean fluorescence of FACs-gated CD8 T cells that were stimulated in the presence or absence of TNF blocking agents (n=8). DNA damage was measured by intracellular staining of phosphorylated gH2AX on FACs gated CD8 T cells in the presence or absence of etanercept (n=17) or infliximab (n=14) (D), or selective blocking antibodies against TNFR1 or TNFR2 (E) (n=12). CD8 T cells were pre-gated on Live+/CD3+/CD4-. Data for A-C are mean ± s.e.m. and P values were calculated using one-way ANOVA with Tukey's multiple comparsisions test. In D and E, the samples were compared by a paired two-tailed t-test.
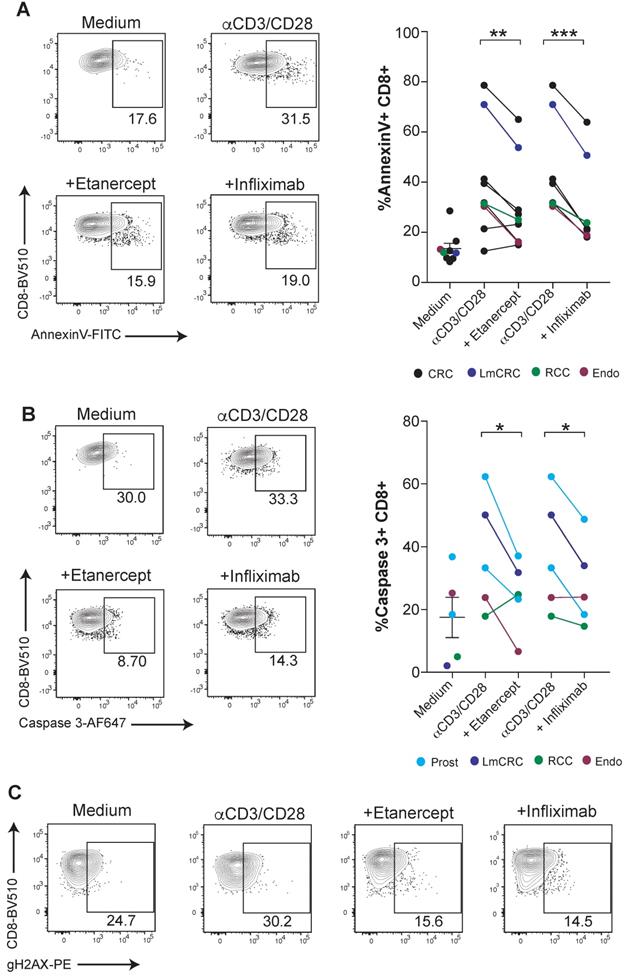
CD8 T-cell mediated immunity is especially relevant for the defense against malignancies and viral infections in humans. Next, we tested if human tumor-infiltrating lymphocytes (TILs) were susceptible to TNF-mediated cell death. For this purpose, fresh TILs obtained from a number of surgical specimens of a variety of cancers were stimulated with anti-CD3/CD28 mAbs in combination with TNF blocking agents. We found that etanercept and infliximab downregulated the surface binding of Annexin V on CD8 T cells and caspase 3 activation (Figure 3A-B). Moreover, tumor-infiltrating CD8 T cells displayed diminished TNF-induced DNA damage, as measured by gH2AX (Figure 3C). These results highlight the potential relevance of TNF-mediated AICD for hindering immunotherapy in cancer patients.
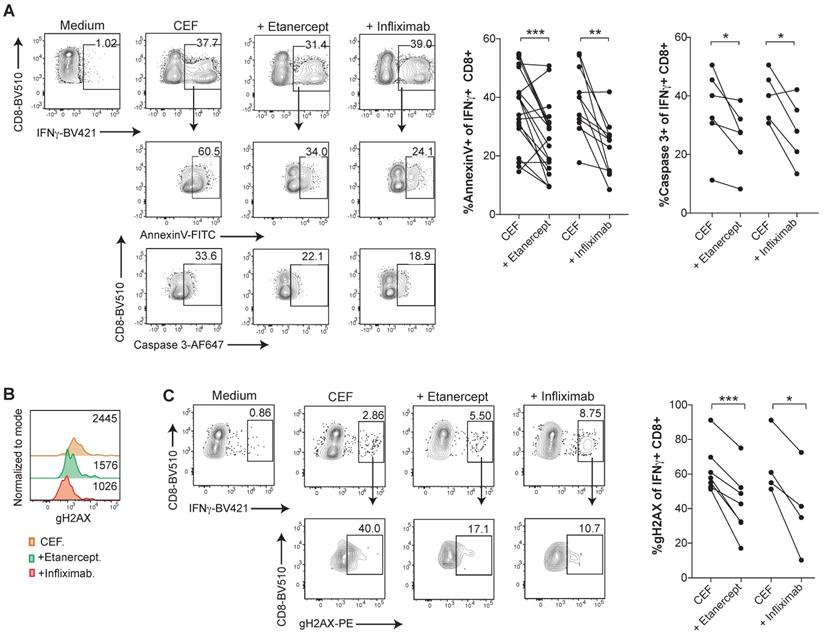
In order to investigate TNF-mediated cell death in an antigen-specific manner in virus-specific CD8 T cell responses, PBMCs were expanded with a pool of viral peptides containing HLA class I-restricted peptides derived from Cytomegalovirus, Epstein-Barr virus, and Influenza virus (CEF) antigens. CEF-specific T cells, identified and gated as IFN-γ+ CD8+ T cells upon incubation with the peptide pools, showed high levels of Annexin V binding and active caspase 3, whereas TNF blockade with etanercept or infliximab significantly reduced lymphocyte death (Figure 4A-B). Similarly, CEF-specific CD8 T cells displayed reduced levels of gH2AX staining in the presence of TNF neutralizing agents (Figure 4C-D).
In summary, our results support the notion in humans that some virus-reactive and tumor-infiltrating CD8 T cells undergo AICD in a TNF-dependent fashion. The use of TNF blocking agents reduces oxidative stress, hyperpolarization of mitochondria, and the generation of DNA damage, features that precede the demise of some activated CD8 T cells. Our observation is in line with other studies showing that AICD in CD8 T cells is mediated by TNFR2 [18,19], whereas other groups have shown that TNFR1 deficient T cells are protected to some extent from TNF-mediated cell death [21,30]. It is likely that the involvement of each TNF receptor in AICD is dependent on the time elapsed since the occurrence of T cell activation and on tissue context. Recent evidence has been published suggesting that TNFR2 stimulation with agonist antibodies is costimulatory for T cells [31,32]. However, under intense signal-1 conditions, it could be derived into AICD.
TNF blockade attenuates AICD in human CD8 tumor-infiltrating lymphocytes. Fresh cell suspensions from surgical tumor specimens were activated with plate-bound anti-CD3 and soluble anti-CD28 mAbs for 4 days in the presence or absence of anti-TNF blocking agents. The type of tumor is color-coded and indicated in the figure legend (CRC: colorectal carcinoma, LmCRC: colorectal carcinoma liver metastasis, RCC: renal cell carcinoma, Endo: endometrial adenocarcinoma, Prost: prostate carcinoma). Apoptotic cell death was measured by surface Annexin V binding (A) and active caspase 3 intracellular staining (B) on tumor-infiltrating CD8 T cells. Data for A; anti-CD3/CD28 + etanercept (n = 9); anti-CD3/CD28 + infliximab (n = 7). Data for B; (n = 5). C, representative dot plots of phosphorylated gH2AX on FACs-gated CD8 T cells from an endometrial cancer in the cultures set up. CD8 T cells were pre-gated on Live+/CD45-/CD3+/CD4- . P values were calculated using a paired two-tailed t-test.
Human CD8 T cells specific for CMV, EBV or Influenza virus are susceptible to TNF-mediated AICD. PBMCs from healthy donors were stimulated for 7 days with a pool of MHC-I restricted peptides from CMV, EBV and Flu virus antigens, in the presence or absence of anti-TNF-blocking agents. A, analysis of surface Annexin V binding and activated caspase 3 staining of intracellular IFN-γ+ CD8+ T cells upon peptide re-stimulation in the presence or abscence of infliximab or etanercept. Data for Annexin V binding: CEF + etanercept (n=17); CEF + infliximab (n=12). Data for activated caspase 3 staining; CEF + etanercept (n=6); CEF + infliximab (n=5). (B and C), flow cytometry assesment of intracellular staining of phosphorylated gH2AX (B) and percentage (C) of IFN-γ+ CD8+ T cells in the cultures set up in the presence or absence of entanercept (n=7) or infliximab (n=4). CD8 T cells were pre-gated on Live+/CD3+/CD4-. P values were calculated using a paired two-tailed t-test.
Discussion
Altogether, these data in cultured human CD8 T cells reveal that the use of TNF blocking agents could improve the viable fate of tumor- and antigen-specific CD8+ T cells following cognate antigen encounter. Our group and others have shown [21,24,33] that blocking TNF in combination with immune checkpoints facilitates an increase in CD8 T cell numbers and viability in the tumor microenvironment and tumor draining lymph nodes, enhancing anti-tumor efficacy while paradoxically reducing immune related adverse effects (irAEs). In a recent report, cancer patients treated with immune checkpoint blockade agents having to receive infliximab for irAE management did not show inferior outcomes of their malignancies [34]. An ongoing phase I clinical trial (NCT03293784) is evaluating the safety and tolerability of treating metastatic melanoma patients with immune checkpoint blockade in combination with the anti-TNF blocking agents infliximab and certolizumab and is expecting to mitigate or avoid irAEs while augmenting antitumor efficacy. Our study showing inhibition of AICD in human CD8 T cells upon TNF blockade points in this direction.
Conclusions
Our study conclusively shows in human CD8 T cells the TNF-mediated AICD phenomena previously described in mouse models and highlights that they can be exploited in immunotherapy since they are relevant for virus-specific and tumor-infiltrating T lymphocytes. Importantly, such effects could be interfered with using clinically available agents such as infliximab and etanercept.
Materials and methods
Subjects and sample collection
This study was approved by the Regional Ethics Committee and written informed consent was obtained from all participants. A total of 25 healthy volunteers participated in the study. PBMCs were separated using Ficoll-Paque Plus (GE Healthcare) density gradient centrifugation. Fresh and sterile tumor tissue samples were obtained from the Department of Pathology of the University Clinic of Navarra, following written, signed, and dated informed consent according to a protocol approved by the institutional ethics committee protocol (2019-76). The characteristics of the patients used in the study are depicted below:
The characteristics of patients
| Gender | Age at surgery | Disease | Stage |
|---|---|---|---|
| Male | 87 | Renal cell carcinoma (RCC) | pT3NxMx |
| Male | 61 | Colorectal carcinoma liver metastasis (LmCRC) | pT3N2M1 |
| Female | 80 | Colorectal carcinoma (CRC) | pT2N0 |
| Female | 80 | Colorectal carcinoma (CRC) | pT3N0Mx |
| Female | 71 | Colorectal carcinoma (CRC) | pTispN0 |
| Female | 62 | Sigmoid adenocarcinoma (CRC) | pT2N0 |
| Male | 60 | Colorectal carcinoma (CRC) | pT3N0 |
| Female | 63 | Endometrial adenocarcinoma (Endo) | pT1bN0 |
| Male | 67 | Prostate cancer (Prost) | pT2cN0 |
| Male | 57 | Prostate cancer (Prost) | pT2bN0 |
Activation-induced cell death assessment in PBMCs
PBMCs from healthy donors were isolated by Ficoll-Hypaque gradient separation (Lymphoprep). PBMC were stimulated with 0.5 µg/ml of plate-bound anti-CD3 mAb (OKT-3; eBioscience) and 1 µg/ml of soluble anti-CD28 mAb in RPMI-10% FBS supplemented with IL-2 (20 U/ml, Proleukin) in the presence or absence of 10 μg/ml of anti-hTNF (Infliximab, Janssen), 4 μg/ml of Etanercept (Sandoz Farma), 1 μg/ml anti-TNFR1 (clone: 55R-170, ThermoFisher Scientific) or 1 μg/ml anti-TNFR2 (clone: 2222.311, ThermoFisher Scientific). Seven days later, cells were re-plated into fresh anti-CD3/CD28 mAbs coated plates and cultured overnight in the same conditions. Cells were analyzed by multicolor flow cytometry (BD FACSCanto™ II system; Zombie NIR, anti-CD3 PeCy7 BD, anti-CD8 BV510, anti-CD4-PE, Annexin V-FITC, anti-CD25-BV421, and anti-PD-1 PerCPCy5.5 (BioLegend). All analyses were performed using Flowjo software (Tree Star).
Activation-induced cell death assessment in viral antigen-specific T cells
Whole PBMCs from healthy donors were stimulated with 0.5 μg/ml CEF extended pool consisting of 32 8-10-mer peptides, each corresponding to a defined HLA class-I restricted epitope from Cytomegalovirus, Epstein-Barr virus and Influenza virus (JPT Peptide Technologies) in the presence of IL-2 (20U/ml) and in the presence or absence of 10 μg/ml of anti-hTNF (Infliximab, Janssen) or 4 μg/ml of Etanercept (Sandoz Farma). On day 7, PBMCs were restimulated using 0.5 μg/ml of CEF peptides under the same conditions and in the presence of brefeldin A (BFA).
Tumor explants
Tumor tissue was digested using enzymes (1 ng/ml of collagenase IV and 0.1 ng/ml of DNAse I) and mechanical maceration before being passed through a 70μm cell strainer. Cell suspensions were stimulated with 0.5 µg/ml of plate-bound anti-CD3 mAb (OKT-3; eBioscience) and 1µg/ml of anti-CD28 mAb in RPMI-10%FBS supplemented with IL-2 (20 U/ml) in the presence or absence of 10 μg/ml of anti-hTNF (Infliximab, Janssen) or 4 μg/ml of Etanercept (Sandoz Farma). On day 4, cells were restimulated in the same culture conditions.
Intracellular and intranuclear staining of human PBMCs
Dead cells were excluded using a live/ dead fixable dye staining kit (BioLegend). Cells were stained for surface markers and then fixed and permeabilized with CytoFix/Perm Buffer (BD). Intracellular molecules were detected using anti-IFN-γ-BV421 (BD) and anti-caspase 3-AF647 (BD) and anti-Granzyme B AF647 (BioLegend). For intranuclear staining, the True nuclear transcription buffer set from Biolegend was used according to the manufacturer protocol. Antibody against anti-Ki67 AF488 (BioLegend) was used. All samples were acquired on a BD Canto II. All analyses were performed using Flowjo software (Tree Star).
Intranuclear staining of gH2AX in human PBMCs
Dead cells were excluded using a live/dead fixable dye staining kit (BioLegend). Cells were stained for surface markers and then fixed with 4% of paraformaldehyde. Cells were then permeabilized with cold Perm III Buffer (BD). Then, gH2AX-PE (BioLegend) was detected by intranuclear staining. All samples were acquired on a BD Canto II. All analyses were performed using Flowjo software (Tree Star).
Intracellular ROS and TMRM measurement
For analysis of intracellular ROS, PBMCs were incubated with 5 μM of CellROS Deep Red reagent (ThermoFisher) for 30 min at 37°C. Membrane potential was assessed using the potentiometric dye tetramethylrhodamine methyl ester (TMRM; Sigma Aldrich) at a final concentration of 125 ng/ml for 30 min at 37°C. Cells were then stained for live/dead and surface markers. All samples were acquired on a BD Canto II. All analyses were performed using Flowjo software (Tree Star).
In vitro killing assay
In vitro real-time killing assays were performed by measuring electric impedance overtime in an Xcelligence Real-Time Cell Analysis Instrument (ACEA). 5x104 HCT116 cells were seeded onto a 16-well plate (ACEA) and cultured overnight in a Xcelligence instrument for cell adhesion and stabilization. After overnight culture, 2.5x105 human primary CD8 T cells were added with 0.5 μg/ml of anti-CD3-Epcam bispecific T-cell engager (BiTE) (Creative Biolabs), in the presence or absence of 1 μg/ml anti-TNFR1 (clone: 55R-170, ThermoFisher Scientific) or 1 μg/ml anti-TNFR2 (clone: 2222.311, ThermoFisher Scientific). Electric impedance was measured every 5 min for 25 h.
Statistical analysis
Statistical analyses were performed using two-way ANOVA, Student's t-tests and Tukey's tests, as appropriate and indicated in each figure. Significant differences were marked on figures legends as *<0.05, **<0.01 and ***<0.001.
Abbreviations
AICD: activation-induced cell death; ANOVA: analysis of variance; DNA: deoxyribonucleic acid; irAEs: immune related adverse effects; CTLA-4: Cytotoxic T-Lymphocyte Antigen 4; FADD: Fas-associated death domain; IFNγ: interferon gamma; PBMCs: peripheral blood mononuclear cells; PD-1: programmed cell death protein 1; TMRM: Tetramethylrhodamine, methyl ester; TILs: tumor infiltrating lymphocytes; TNF: tumor necrosis factor; TNFR1: tumor necrosis factor receptor 1; TNFR2: tumor necrosis factor receptor 2; TRADD: TNFR1-associated domain protein.
Supplementary Material
Supplementary figure.
Acknowledgements
This work was supported by Spanish Ministry of Economy and Competitiveness (MINECO SAF2014-52361-R and SAF 2017-83267-C2-1R [AEI/FEDER, UE]), Cancer Research Institute (CRI), Asociación Española Contra el Cancer (AECC) Foundation under Grant GCB15152947MELE, Joint Translational Call for Proposals 2015 (JTC 2015) TRANSCAN-2 (code: TRS-2016-00000371), Fondo de Investigación Sanitaria-Fondo Europeo de Desarrollo Regional (FEDER) under Grants PI14/01686, PI13/00207, PI16/00668, PI19/01128 and H2020 PROCROP project under Grant 635122. M.A. is supported by the Marie Skłodowska-Curie fellowship (CINK 746985). AT has received financial support through “la Caixa” Banking Foundation (LCF/BQ/LR18/11640014). Esther Guirado is acknowledged for project managing, Dr. Diego Aligani for excellent flow cytometry assistance and Dr. Paul Miller for English editing. We are very grateful to all patients and control volunteers who participated in this study and to all clinical staff who helped with participant recruitment. The figures from the graphical abstract contain elements from Servier Medical Art (https://smart.servier.com/), licensed under Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/).
Author Contributions
I.O., P.B. and I.M. designed experiments. I.O., I.M., C.M. and A.A performed the experiments and processed human samples. C.D.A. and M.S. provided fresh human samples. I.O. and P.B performed all statistical analyses. I.O., L.M., M.A., M.C.O., I.E., A.T., P.B. and I.M. analyzed the data. I.O., P.B. and I.M. wrote the manuscript. All authors performed a critical revision of the manuscript for important intellectual content and final approval of the manuscript.
Competing Interests
I.M. reports advisory roles with Roche-Genentech, Bristol-Myers Squibb, CYTOMX, Incyte, MedImmune, Tusk, F-Star, Genmab, Molecular Partners, Alligator, Bioncotech, MSD, Merck Serono, Boehringer Ingelheim, Astra Zeneca, Numab, Catalym, and Bayer, and research funding from Roche, BMS, Alligator, and Bioncotech. P.B. reports advisory roles with Tusk and Moderna, research funding from Sanofi, and Bavarian Nordic and speaker honoraria from BMS, MSD, Novartis and AstraZeneca. The rest of the authors have no conflict of interest to declare.
References
1. Razvi ES, Jiang Z, Woda BA, Welsh RM. Lymphocyte apoptosis during the silencing of the immune response to acute viral infections in normal, lpr, and Bcl-2-transgenic mice. Am J Pathol. 1995;147:79-91
2. Webb S, Morris C, Sprent J. Extrathymic tolerance of mature T cells: clonal elimination as a consequence of immunity. Cell. 1990;63:1249-56
3. MacDonald HR, Baschieri S, Lees RK. Clonal expansion precedes anergy and death of V beta 8+ peripheral T cells responding to staphylococcal enterotoxin B in vivo. Eur J Immunol. 1991;21:1963-6
4. Badovinac VP, Tvinnereim AR, Harty JT. Regulation of antigen-specific CD8+ T cell homeostasis by perforin and interferon-gamma. Science. 2000;290:1354-8
5. D'Adamio L, Awad KM, Reinherz EL. Thymic and peripheral apoptosis of antigen-specific T cells might cooperate in establishing self tolerance. Eur J Immunol. 1993;23:747-53
6. Kabelitz D, Pohl T, Pechhold K. Activation-induced cell death (apoptosis) of mature peripheral T lymphocytes. Immunol Today. 1993;14:338-9
7. Chen Y, Inobe J, Marks R, Gonnella P, Kuchroo VK, Weiner HL. Peripheral deletion of antigen-reactive T cells in oral tolerance. Nature. 1995;376:177-80
8. Moskophidis D, Lechner F, Pircher H, Zinkernagel RM. Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature. 1993;362:758-61
9. Ju ST, Panka DJ, Cui H, Ettinger R, el-Khatib M, Sherr DH. et al. Fas(CD95)/FasL interactions required for programmed cell death after T-cell activation. Nature. 1995;373:444-8
10. Alderson MR, Tough TW, Davis-Smith T, Braddy S, Falk B, Schooley KA. et al. Fas ligand mediates activation-induced cell death in human T lymphocytes. J Exp Med. 1995;181:71-7
11. Fortner KA, Budd RC. The death receptor Fas (CD95/APO-1) mediates the deletion of T lymphocytes undergoing homeostatic proliferation. J Immunol. 2005;175:4374-82
12. Salmon M, Pilling D, Borthwick NJ, Viner N, Janossy G, Bacon PA. et al. The progressive differentiation of primed T cells is associated with an increasing susceptibility to apoptosis. Eur J Immunol. 1994;24:892-9
13. Groux H, Torpier G, Monte D, Mouton Y, Capron A, Ameisen JC. Activation-induced death by apoptosis in CD4+ T cells from human immunodeficiency virus-infected asymptomatic individuals. J Exp Med. 1992;175:331-40
14. Katsikis PD, Wunderlich ES, Smith CA, Herzenberg LA, Herzenberg LA. Fas antigen stimulation induces marked apoptosis of T lymphocytes in human immunodeficiency virus-infected individuals. J Exp Med. 1995;181:2029-36
15. Ware CF, Crowe PD, Vanarsdale TL, Andrews JL, Grayson MH, Jerzy R. et al. Tumor necrosis factor (TNF) receptor expression in T lymphocytes. Differential regulation of the type I TNF receptor during activation of resting and effector T cells. J Immunol. 1991;147:4229-38
16. Hsu H, Xiong J, Goeddel DV. The TNF receptor 1-associated protein TRADD signals cell death and NF-kappa B activation. Cell. 1995;81:495-504
17. Rothe M, Sarma V, Dixit VM, Goeddel DV. TRAF2-mediated activation of NF-kappa B by TNF receptor 2 and CD40. Science. 1995;269:1424-7
18. Zheng L, Fisher G, Miller RE, Peschon J, Lynch DH, Lenardo MJ. Induction of apoptosis in mature T cells by tumour necrosis factor. Nature. 1995;377:348-51
19. Alexander-Miller MA, Derby MA, Sarin A, Henkart PA, Berzofsky JA. Supraoptimal peptide-major histocompatibility complex causes a decrease in bc1-2 levels and allows tumor necrosis factor alpha receptor II-mediated apoptosis of cytotoxic T lymphocytes. J Exp Med. 1998;188:1391-9
20. Herbein G, Mahlknecht U, Batliwalla F, Gregersen P, Pappas T, Butler J. et al. Apoptosis of CD8+ T cells is mediated by macrophages through interaction of HIV gp120 with chemokine receptor CXCR4. Nature. 1998;395:189-94
21. Bertrand F, Rochotte J, Colacios C, Montfort A, Tilkin-Mariame AF, Touriol C. et al. Blocking Tumor Necrosis Factor alpha Enhances CD8 T-cell-Dependent Immunity in Experimental Melanoma. Cancer Res. 2015;75:2619-28
22. Pimentel-Muinos FX, Seed B. Regulated commitment of TNF receptor signaling: a molecular switch for death or activation. Immunity. 1999;11:783-93
23. Fotin-Mleczek M, Henkler F, Samel D, Reichwein M, Hausser A, Parmryd I. et al. Apoptotic crosstalk of TNF receptors: TNF-R2-induces depletion of TRAF2 and IAP proteins and accelerates TNF-R1-dependent activation of caspase-8. J Cell Sci. 2002;115:2757-70
24. Perez-Ruiz E, Minute L, Otano I, Alvarez M, Ochoa MC, Belsue V. et al. Prophylactic TNF blockade uncouples efficacy and toxicity in dual CTLA-4 and PD-1 immunotherapy. Nature. 2019;569:428-32
25. Montfort A, Colacios C, Levade T, Andrieu-Abadie N, Meyer N, Segui B. The TNF Paradox in Cancer Progression and Immunotherapy. Front Immunol. 2019;10:1818
26. Meier B, Radeke HH, Selle S, Younes M, Sies H, Resch K. et al. Human fibroblasts release reactive oxygen species in response to interleukin-1 or tumour necrosis factor-alpha. Biochem J. 1989;263:539-45
27. Shoji Y, Uedono Y, Ishikura H, Takeyama N, Tanaka T. DNA damage induced by tumour necrosis factor-alpha in L929 cells is mediated by mitochondrial oxygen radical formation. Immunology. 1995;84:543-8
28. Ehrenberg B, Montana V, Wei MD, Wuskell JP, Loew LM. Membrane potential can be determined in individual cells from the nernstian distribution of cationic dyes. Biophys J. 1988;53:785-94
29. Teijeira A, Labiano S, Garasa S, Etxeberria I, Santamaria E, Rouzaut A. et al. Mitochondrial Morphological and Functional Reprogramming Following CD137 (4-1BB) Costimulation. Cancer Immunol Res. 2018;6:798-811
30. Speiser DE, Sebzda E, Ohteki T, Bachmann MF, Pfeffer K, Mak TW. et al. Tumor necrosis factor receptor p55 mediates deletion of peripheral cytotoxic T lymphocytes in vivo. Eur J Immunol. 1996;26:3055-60
31. Williams GS, Mistry B, Guillard S, Ulrichsen JC, Sandercock AM, Wang J. et al. Phenotypic screening reveals TNFR2 as a promising target for cancer immunotherapy. Oncotarget. 2016;7:68278-91
32. Tam EM, Fulton RB, Sampson JF, Muda M, Camblin A, Richards J. et al. Antibody-mediated targeting of TNFR2 activates CD8(+) T cells in mice and promotes antitumor immunity. Sci Transl Med. 2019 11
33. Bertrand F, Montfort A, Marcheteau E, Imbert C, Gilhodes J, Filleron T. et al. TNFalpha blockade overcomes resistance to anti-PD-1 in experimental melanoma. Nat Commun. 2017;8:2256
34. Badran YR, Cohen JV, Brastianos PK, Parikh AR, Hong TS, Dougan M. Concurrent therapy with immune checkpoint inhibitors and TNFalpha blockade in patients with gastrointestinal immune-related adverse events. J Immunother Cancer. 2019;7:226
Author contact
![]() Corresponding author: Ignacio Melero (E-mail: imeleroes) and Itziar Otano (E-mail: iotanoanunav.es), Cima Universidad de Navarra, Avenida Pio XII 55, 31008 Pamplona Spain. Phone number: +34948194700; Fax number: +34948194717.
Corresponding author: Ignacio Melero (E-mail: imeleroes) and Itziar Otano (E-mail: iotanoanunav.es), Cima Universidad de Navarra, Avenida Pio XII 55, 31008 Pamplona Spain. Phone number: +34948194700; Fax number: +34948194717.
 Global reach, higher impact
Global reach, higher impact