13.3
Impact Factor
Theranostics 2020; 10(10):4323-4333. doi:10.7150/thno.43251 This issue Cite
Research Paper
Aristolochic acid mutational signature defines the low-risk subtype in upper tract urothelial carcinoma
1. Key Laboratory of Genomics and Precision Medicine, Beijing Institute of Genomics, Chinese Academy of Sciences, Beijing 100101, China.
2. Department of Urology, Peking University First Hospital, Beijing 100034, China.
3. University of Chinese Academy of Sciences, Beijing 100049, China.
4. Institute of Urology, Peking University, Beijing 100034, China.
5. National Urological Cancer Center, Beijing, 100034, China.
6. Department of Urology, The Third Hospital of Hebei Medical University, Hebei 050051, China.
7. Jamil-ur-Rahman Center for Genome Research, PCMD, ICCBS, University of Karachi, Pakistan.
8. Institute of Stem cell and Regeneration, Chinese Academy of Sciences, Beijing 100101, China.
*These authors contributed equally to this work.
Abstract
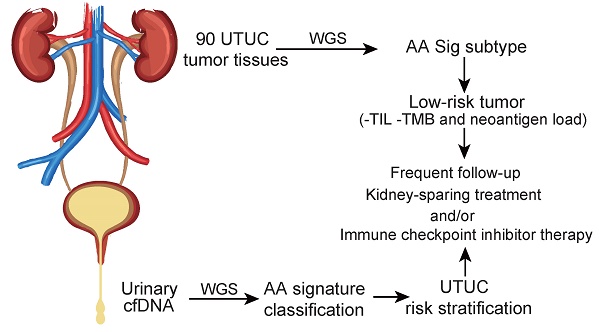
Rationale: Dietary exposure to aristolochic acids and similar compounds (collectively, AA) is a significant risk factor for nephropathy and subsequent upper tract urothelial carcinoma (UTUC). East Asian populations, who have a high prevalence of UTUC, have an unusual genome-wide AA-induced mutational pattern (COSMIC signature 22). Integrating mutational signature analysis with clinicopathological information may demonstrate great potential for risk ranking this UTUC subtype.
Methods: We performed whole-genome sequencing (WGS) on 90 UTUC Chinese patients to extract mutational signatures. Genome sequencing data for urinary cell-free DNA from 26 UTUC patients were utilized to noninvasively identify the mutational signatures. Genome sequencing for primary tumors on 8 out of 26 patients was also performed. Metastasis-free survival (MFS) and cancer-specific survival (CSS) were measured using Kaplan-Meier methods.
Results: Data analysis showed that a substantial proportion of patients harbored the AA mutational signature and were associated with AA-containing herbal drug intake, female gender, poor renal function, and multifocality. Field cancerization was found to partially contribute to multifocality. Nevertheless, AA Sig subtype UTUC patients exhibited favorable outcomes of CSS and MFS compared to the No-AA Sig subtype. Additionally, AA Sig subtype patients showed a higher tumor mutation burden, higher numbers of predicted neoantigens, and infiltrating lymphocytes, suggesting the potential for immunotherapy. We also confirmed the AA signature in AA-treated human renal tubular HK-2 cells. Notably, the AA subtype could be ascertained using a clinically applicable sequencing strategy (low coverage) in both primary tumors and urinary cell-free DNA as a basis for therapy selection.
Conclusion: The AA mutational signature as a screening tool defines low-risk UTUC with therapeutic relevance. The AA mutational signature, as a molecular prognostic marker using either ureteroscopy and/or urinary cell-free DNA, is especially useful for diagnostic uncertainty when kidney-sparing treatment and/or immune checkpoint inhibitor therapy were considered.
Keywords: upper tract urothelial carcinoma, whole-genome sequencing, mutational signature, aristolochic acids, clinical outcome
 Global reach, higher impact
Global reach, higher impact