13.3
Impact Factor
Theranostics 2020; 10(9):3849-3866. doi:10.7150/thno.39706 This issue Cite
Review
Aptamer-guided extracellular vesicle theranostics in oncology
1. School of Medicine and Centre for Molecular and Medical Research, Deakin University, Waurn Ponds, Victoria, Australia
2. Division of Genetics, Department of Medicine, Brigham and Women's Hospital/Harvard Medical School, 77 Avenue Louise Pasteur, Boston, MA 02115, USA
3. Department for Management of Science and Technology Development, Ton Duc Thang University, Ho Chi Minh City, Vietnam
4. Faculty of Pharmacy, Ton Duc Thang University, Ho Chi Minh City, Vietnam
5. Translational Medical Center, The Chinese People's Liberation Army General Hospital, 28 Fuxing Road, Haidian District, Beijing, China, 100853
6. Institute for Frontier Materials, Deakin University, Waurn Ponds, Victoria, 3216, Australia
7. School of Medicine, Faculty of Medicine, Nursing and Health Sciences, Monash University, 27 Rainforest Walk, Clayton VIC 3800, Australia
8. Department of Pathology, The First Affiliated Hospital, Zhengzhou University, He'nan Key Laboratory of Tumor Pathology, Zhengzhou 450052, China
9. Cancer Care Centre, St George Hospital, Kogarah, and St George and Sutherland Clinical School, University of New South Wales, Kensington, NSW, Australia
10. Laboratory of Tumor Molecular and Cellular Biology, College of Life Sciences, Shaanxi Normal University, 620 West Chang'an Avenue, Xi'an, Shaanxi 710119, China
11. CAS Key Laboratory of Nano-Bio Interface, Suzhou Institute of Nano-Tech and Nano-Bionics, Chinese Academy of Sciences, Suzhou 215123, China
12. Clinical School, Luohe Medical College, 148, Daxue Road, Luohe City, Henan Province, 462000, China
13. Department of General Surgery, Second Affiliated Hospital of Soochow University, 1055 Sanxiang Road, Suzhou, P.R. China, 215004
14. GenePharma-Deakin Joint Laboratory of Aptamer Medicine, Suzhou 215123, China and Waurn Ponds, Victoria 3216, Australia
*These authors contributed equally to this work.
Received 2019-8-27; Accepted 2019-12-20; Published 2020-2-21
Abstract
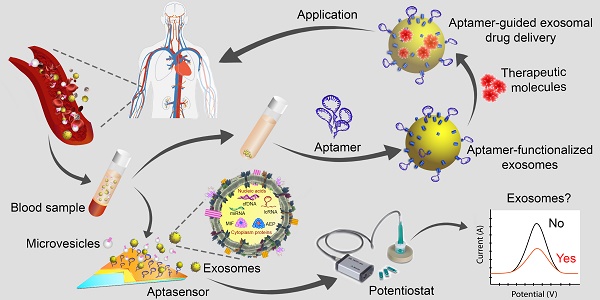
In the past decade, the study of exosomes, nanosized vesicles (50-150 nm) released into the extracellular space via the fusion of multivesicular bodies with the plasma membrane, has burgeoned with impressive achievements in theranostics applications. These nanosized vesicles have emerged as key players in homeostasis and in the pathogenesis of diseases owing to the variety of the cargos they can carry, the nature of the molecules packaged inside the vesicles, and the robust interactions between exosomes and target cells or tissues. Accordingly, the development of exosome-based liquid biopsy techniques for early disease detection and for monitoring disease progression marks a new era of precision medicine in the 21st century. Moreover, exosomes possess intrinsic properties - a nanosized structure and unique "homing effects" - that make them outstanding drug delivery vehicles. In addition, targeted exosome-based drug delivery systems can be further optimized using active targeting ligands such as nucleic acid aptamers. Indeed, the aptamers themselves can function as therapeutic and/or diagnostic tools based on their attributes of unique target-binding and non-immunogenicity. This review aims to provide readers with a current picture of the research on exosomes and aptamers and their applications in cancer theranostics, highlighting recent advances in their transition from the bench to the clinic.
Keywords: exosomes, aptamers, theranostics, liquid biopsy, targeting
Exosomes, a new generation of theranostics
Extracellular vesicles (EVs) are membrane-bound bionanoparticles secreted by all living cells into the extracellular space [1]. Based on their mode of biogenesis and size, EVs are classified as exomeres, exosomes, microvesicles, oncosomes or apoptotic bodies [2, 3]. From humble beginnings as “platelet-dust” half a century ago [4], EVs have emerged as key mediators for in vivo communication among cells, tissues and cross-kingdom molecules [2, 5]. Several decades of biochemical and cell biological investigations have culminated in recent works defining exosomes as 50 to 150 nm in size with surface tetraspanins (CD63, CD81, and CD9) as biomarkers and microvesicles as 100 to 1000 nm in size with annexin A1 as a distinct biomarker [6, 7]. In fact, exosomes circulating in various cell types have been found in the blood and other body fluids with cargos inherited from the cells of origin. These advances have laid the foundation for exosomes to be a novel source of biomarker discovery. Exosomes are known to carry a number of marker proteins, including heat shock proteins (HSPs), tumor-susceptibility gene 101 (Tsg101), the endosomal sorting complex required for transport (ESCRT-3) binding protein Alix, and major histocompatibility complex (MHC) class I and MHC class II complexes [8, 9]. Notably, integrins and other adhesion molecules on the surface of exosomes, such as intercellular adhesion molecule-1 (ICAM-1, also known as CD54) and lymphocyte function-associated antigen 1 (LFA-1) integrin, as well as the exosomal lipid content may facilitate exosome adhesion and fusion with the plasma membrane of recipient cells [10, 11]. In addition, the enrichment of specific transmembrane proteins, such as epidermal growth factor receptors (EGFRs) and epithelial cell adhesion molecule (EpCAM), in exosomes reflects their cellular origin [12, 13]. These proteins are associated with the normal physiology and pathogenesis of many diseases, leading to their utilization as valuable biomarkers [14]. The membranes of exosomes are highly enriched with lipid rafts, which render exosomes highly stable under various in vivo and in vitro conditions [15]. Given the unique lipid composition of their membrane, when compared with that of the cells from which they were derived, exosomes can effectively protect their cargos, such as proteins, mRNA, miRNA, long-noncoding RNA and small nuclear RNA. In addition to their pivotal roles in normal physiology and the pathogenesis of many diseases, exosomes are now poised to become promising next-generation diagnostic and therapeutic tools [16] (Fig. 1).
Extracellular vesicles as diagnostic markers and next-generation therapeutics. Exosomes sized 50 to 150 nm are released from most cell types upon fusion of an intermediate endocytic compartment, the multivesicular body, with the plasma membrane. Microvesicles are released by direct budding from the cell surface. Both types of vesicles are composed of an aqueous core and a lipid bilayer membrane and contain a variety of proteins, DNAs, RNAs, lipids and other metabolites. Image of the circulatory system was modified from https://commons.wikimedia.org/wiki/File:Circulatory_System_en.svg by LadyofHats, with permission.
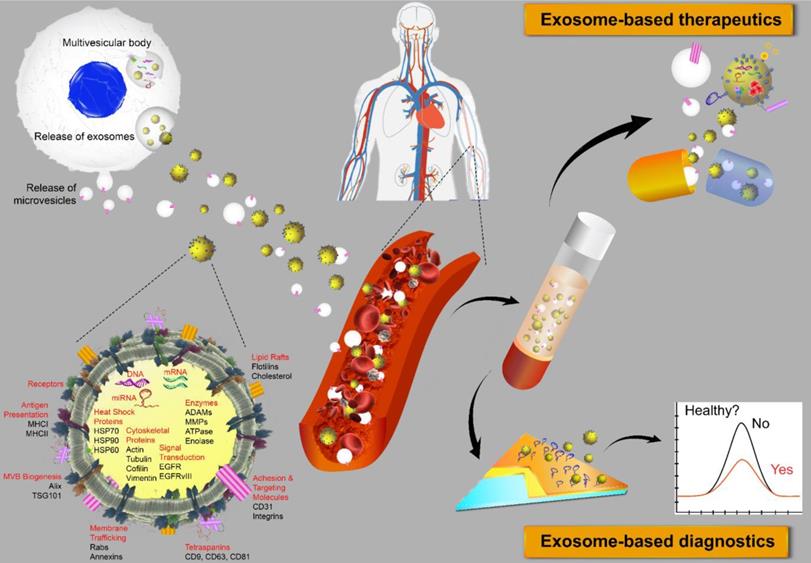
Indeed, exosomes carry information on not only their cells of origin, thus providing readily accessible diagnostic markers, but also the progression and prognosis of a particular disorder. For instance, cancer cell-derived exosomes can carry membrane proteins involved in cancer progression. A recent study on programmed death-ligand 1 (PD-L1) in exosomes from metastatic melanoma cells found that exosomal PD-L1 could inhibit CD8 T cells and facilitate tumor growth [17]. Another recent study demonstrated that integrins such as α6, αv and β1, found on cancer cell-derived exosomes could be used to distinguish between different types of cancer, such as breast, kidney, colon and ovarian cancers, and to predict tumor stage, as higher levels of these proteins on exosomes were secreted from the more aggressive progenitor cancer cells [18]. Moreover, miRNAs extracted from exosomes can be used as signatures for disease detection and as indicators of the tissue of origin not only in cancer [19] but also in other diseases such as neurodegenerative diseases [20], obesity [21], diabetes [22], lupus nephritis [23], cardiovascular diseases [24], and lung diseases [25]. It has been shown that at least some exosomes are able to cross the blood-brain barrier. Thus, the content and number of exosomes in the cerebrospinal fluid and plasma may provide valuable information on the pathogenesis and progression of neurodegenerative diseases [26]. In a recent study, plasma exosomes from subjects with HIV-associated neurological disorders and from healthy subjects were isolated using antibodies against the neuronal cell adhesion molecule L1 (L1CAM). The concentration of L1CAM+ neuronal exosomes was found to be lower in the neurocognitively impaired participants than that in the subjects without neurological impairment. In addition, increased levels of the neuronal markers neurofilament light (NfL) and synaptophysin were found in the neuron-derived exosomes in plasma from HIV-infected individuals [27]. β-amyloid (Aβ) plaques have been regarded as among the earliest hallmarks of the disease, and exosomal β-amyloid in the bloodstream has been utilized for the detection of Alzheimer's disease [28]. In addition, CD63/CD66b and CD63/MUC-1 double-positive exosomes in colonic luminal fluid aspirates from patients with inflammatory bowel disease were found to be of neutrophil and epithelial cell origin, respectively, and thus have been utilized as potential fecal biomarkers for mucosal bowel inflammation [29]. Interestingly, this dual positive exosome-based approach has also been applied in the development of novel methods for assessing placenta health via the analysis of circulating syncytiotrophoblast exosomes released from the placenta surface that directly entered maternal circulation [30]. Exosomes in the circulation that were positive for both a placenta-specific marker (PLAP) and an exosome marker, CD63, were found to gradually increase after 6 weeks gestation, indicating that the release of syncytiotrophoblast exosomes into maternal circulation occurs before the onset of blood flow into the intervillous space, which occurs at approximately 10 weeks gestation in a normal pregnancy [30-32]. Furthermore, exosomes harboring proteins involved in innate immunity in saliva and tears were also explored as potential biomarkers of primary Sjögren's syndrome [33], demonstrating the versatile use of exosomes in biofluids as biomarkers for staging and monitoring diseases.
The biological features of exosomes endow these vesicles with the ability to efficaciously deliver therapeutic molecules. The “homing effect” of exosomes indicates that exosomal therapeutics delivered are likely to induce more pronounced anticancer effects in cancer cells from which the exosomes are derived [34]. There is also evidence suggesting that mesenchymal stromal cell (MSC)-derived exosomes constitute a promising new modality for the treatment of stroke and traumatic brain injury. In this context, exosomes loaded with selected miRNAs can alter the biological functions of recipient cells [35]. Some clinical trials involving exosomes were recently registered, mostly in the contexts of stroke, diabetes, cutaneous wounds and cancer [35]. Undoubtedly, we will witness an increasing number of exosome-based therapies in the next few years once the biological functions, molecular mechanism(s) and the safety of exosomes, as well as their manufacturing and associated quality control procedures, are clarified and developed thoroughly.
Exosome-based liquid biopsy in precision oncology
The survival and quality of life of patients with cancer can be significantly improved when the disease is diagnosed at an early stage. Thus, the early detection of cancer is pivotal for improving treatment outcomes and reducing the cancer burden. The methodologies used for early cancer detection should be sensitive, accurate, minimally invasive and able to be repeatedly performed on demand. To mitigate the limitations of traditional tissue biopsy, a liquid biopsy of biological fluids, such as blood or urine, has been utilized to detect cancer biomarkers with minimal invasiveness, making this technique suitable for obtaining multiple samples over time [36-38]. The successful clinical translation of liquid biopsies is exemplified by CellSearch®, a diagnostic test for cancer metastasis that detects circulating cancer cells in the blood, which was approved by U.S. Food and Drug Administration (FDA) in 2004. Liquid biopsy is also indispensable for monitoring therapeutic effectiveness in cancer patients undergoing treatment, as well as for providing real-time feedback and guidance on adjustments to optimize treatment regimens. The presence of EVs released by cancer cells in bodily fluids makes them accessible for simple and repeated sampling. Moreover, in some cases, cancer cells release more exosomes into plasma than do healthy cells, and a number of cancer-related biomarkers have been shown to be overexpressed in these cancer-derived exosomes [39, 40]. Exosome-associated membrane proteins are valuable for the isolation of exosomes and the characterization of their biological functions in cancer progression [37, 41]. The importance of developing potentially novel exosome-based cancer biomarkers has been extensively exploited. The amounts of specific proteins in exosomes, such as glypican 1 [42, 43], may constitute biochemical signatures for determining the stage of cancer. Indeed, elevated glypican-1 in circulating exosomes can be used for the early diagnosis of pancreatic cancer as the amount of exosomal glypican-1 correlates with tumor burden [42, 43]. In addition, the TYRP2 levels in exosomes were found to be significantly increased in the plasma of patients with stage III melanoma who eventually developed metastases [44]. Moreover, L1CAM, CD24 and extracellular matrix metalloproteinase inducers were shown to be increased in exosomes released from ovarian cancer cells in both malignant ascites and serum [45].
One interesting target protein for liquid biopsy development is EpCAM [46]. Elevated EpCAM levels are associated with increased cell proliferation, tumor development and progression, as well as with reduced overall survival of cancer patients [47]. As an epithelial marker, EpCAM is expressed at low levels in all epithelial cells. The blood of healthy subjects contains very few EpCAM-positive exosomes or EVs [48-50]. However, when normal epithelial cells transform into carcinoma cells, significantly more EpCAM-positive EVs are detected in both the general circulation and in other body fluids, such as pleural effusions [51] in patients with carcinomas [52]. Hence, circulating EpCAM-positive exosomes could be a minimally invasive diagnostic marker for the early detection of cancers of epithelial origin or carcinomas. Indeed, EpCAM-positive exosomes were harvested from the serum and/or the plasma of lung, ovarian and colorectal cancer patients using an anti-EpCAM antibody to establish novel cancer diagnostic methods [53-55]. While EpCAM-positive exosomes can be detected in both patients with benign ovarian disease and ovarian cancer, the exosomal miRNA profiles were remarkably distinct in cancer patients and patients with benign disease [53]. Interestingly, the miRNAs found in the exosomes from ovarian cancer patients were abundant and similar among patients, but they were not be detected in healthy subjects [53]. Similar results were found regarding the profiles of lung cancer patients and normal controls [54] and of colorectal cancer patients and healthy individuals [55]. Of note, miRNAs encapsulated by exosomes are remarkably stable in circulation because exosomes can protect miRNA against RNase-mediated degradation [55]. miRNA profiling has emerged as a robust and promising strategy for exosome-based diagnostics, as PCR-based miRNA profiling requires a much lower concentration of exosome than is required for protein-based exosome analysis [55]. Indeed, several research groups demonstrated that the association of miRNAs with circulating cancer- derived exosomes could be used as a signature for cancer diagnosis. For example, analyses of exosomal miRNAs revealed a differential expression pattern in patients with prostate cancer compared to controls. Specifically, in the prostate cancer samples, the levels of miR-100-5p and miR-21-5p were found to be valuable for evaluating prostate cancer progression and metastasis [56]. Furthermore, tumor-derived exosomal miRNAs, including the adenocarcinoma- specific miR-181-5p, miR-30a-3p, miR-30e-3p and miR-361-5p and the squamous cell carcinoma-specific miR-10b-5p, miR-15b-5p and miR-320b, have been shown to be potential biomarkers useful for distinguishing adenocarcinoma from squamous cell carcinoma in the early diagnosis of non-small cell lung cancer [57]. Most recently, a research group from Spain analyzed exosomal miRNA levels in serum samples from 53 women with breast cancer who had been initially diagnosed with localized breast cancer and who were receiving neoadjuvant chemotherapy, before and during the therapy, and compared the levels to those in eight healthy controls [58]. Exosomal miRNA-21 and miRNA-21-105 were found to be increased in the metastatic patients compared to the levels in the non-metastatic patients and the healthy donors before receiving neoadjuvant therapy. Importantly, the levels of miRNA-21 were found to be directly correlated with the tumor size during neoadjuvant therapy [58]. These intensive studies have culminated in a milestone in exosome-based cancer diagnostics: the FDA approval of the ExoDxTM Prostate IntelliScore (EPI) test in June 2019. ExoDxTM, which is an exosomal RNA-based clinical liquid biopsy test based on a simple urine sample that provides a more precise diagnosis of prostate cancer [59-61].
Comparison of ExoScreen and the conventional method of biomarker detection. ExoScreen can be performed without a prior purification step. This system uses photosensitizer beads composed of streptavidin-coated donor beads to capture biotinylated antibodies in the analyte, and these beads are excited with a laser at 680 nm. Singlet oxygen is released and excites the acceptor beads, which are conjugated to a second antibody recognizing an epitope in the analyte, to amplify the fluorescence signal. This system emits light at 615 nm only when (i) exosomes are captured by both antibodies (CD147 and CD9) and (ii) the exosomes are smaller than 200 nm. Images of the plate reader and immunoblotting technique were modified from https://www.perkinelmer.com/category/microplate-readers, and https://www.bioradiations.com/the-how-and-why-of-normalizing-your-western-blots/, respectively, with permissions.
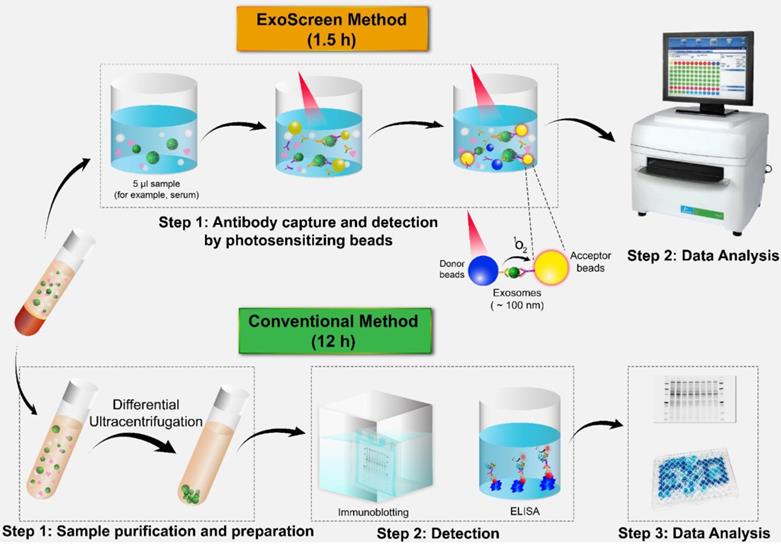
In the past few years, several techniques have been established for the detection of a variety of proteins in exosomes and tumor-associated markers through liquid biopsy. ExoTEST is an enzyme-linked immunosorbent assay (ELISA)-based technique that captures and quantifies exosomes in plasma and other biological fluids by detecting CD63 and Rab5b proteins on exosomes and caveolin-1, a tumor-associated marker [62]. In a clinical study of ExoTEST, high levels of exosomes expressing CD63 and caveolin-1 were detected in the plasma of melanoma patients compared with that in the plasma of healthy controls [39]. Although exosomes released from cancer cells are thought to promote tumor growth and metastasis, their low abundance in the circulation in the early stages of cancer development constitutes a formidable challenge to the use of liquid biopsy. Nevertheless, employing multiple exosomal biomarkers, Yoshioka and colleagues employed multiple exosomal biomarkers to develop an antibody-based ExoScreen technique (Fig. 2) that can be used to monitor CD147-CD9 double-positive exosomes secreted from colorectal cancer cells in only 5 μL of patient serum [63]. Interestingly, this method does not require a prior purification step. Thus, ExoScreen is a highly sensitive and rapid liquid biopsy technique for the detection of disease-specific exosomes in circulation.
The inherent limitations of conventional exosome detection methods include the prolonged time, requirement for large amounts of exosomes and extensive post-labeling processes [64]. To address these issues, high-throughput approaches have been developed that use nanoplasmonic sensors for label-free detection, molecular profiling and multiplexed phenotyping of exosomal proteins [13, 65]. Label-free detection is a portable process that enables point-of-care analyses. For example, a nanoplasmonic exosome (nPLEX) assay was developed based on transmission surface plasmon resonance (SPR) and arrays of periodic nanoholes functionalized with antibodies to identify exosomal CD24 and EpCAM expression in ascites fluid from ovarian cancer patients [13]. In addition, a high-throughput-based molecular profiling technique for use with exosomes was established in the format of an exosome array, in which exosomes were first immobilized using antibodies against CD9/CD81/CD63 imprinted on coated glass slides. Subsequently, a panel of antibodies against 21 different cell surface antigens and cancer antigens was added. This method has been successfully applied for exosome profiling using 1-10 µL of plasma from healthy controls and patients with non-small cell lung carcinoma [65, 66]. Most recently, Zhang et al. enhanced the efficiency and speed of exosome capture using an ultrasensitive biosensor based on a self-assembled 3D herringbone nanoporous structure; the greater surface area of this sensor increased the number of exosomes that could contact the antibody-coated sensing surface [67]. This microfluidic chip was used for the rapid detection of exosomes expressing CD24, EpCAM and folate receptor α (FRα) in only 2 μL of plasma from ovarian cancer patients and healthy controls. This experiment revealed that FRα is a potential biomarker for the early detection and monitoring of ovarian cancer. In addition, the negative charge of exosomes was leveraged to promote electrostatic interactions with nickel at pH values above 5 under physiological conditions. Based on this principle, a simple yet robust strategy for the rapid purification of EVs was developed by combining nickel-based isolates with an amplified luminescent proximity homogeneous assay or droplet digital PCR. This strategy further evolved into an EV-based liquid biopsy used to probe tumor heterogeneity [68]. This method was applied to detect picomolar concentrations of exosomal prostate-specific membrane antigen (PSMA) in the plasma of prostate cancer patients. Impressively, the somatic BRAF and KRAS mutations in exosomes circulating in the plasma of metastatic colorectal cancer patients detected by this technique matched the tissue diagnostics with 100% concordance [68]. Another new exosomal profiling platform developed by Zhang and coworkers is the ExoProfile chip. This device was constructed of 3D porous serpentine nanostructures via patterned colloidal self-assembly to increase the number of reaction sites and improve the exosome biosensing efficiency. Hence, this device detects a panel of surface protein markers on exosomes, including EGFR, human epidermal growth factor receptor 2 (HER2), CA125, FRα, CD24, EpCAM, CD9 and CD63, with high sensitivity in a multiplexed manner [69]. This platform was applied to detect circulating EVs in only 10 μL of plasma from ovarian cancer patients in 3 hours, and the results demonstrated improved accuracy in differentiating early stage from late stage cancer [69]. For early cancer detection, Ramshani and colleagues developed a novel platform for the quantification of both free-floating miRNAs and EV-bound miRNAs in plasma, in which a surface acoustic wave lyses exosomes on a microfluidic chip integrated with another concentration-sensing chip that uses an electrokinetic membrane sensor to measure non-equilibrium ionic currents [70]. This microfluidic chip is a convenient tool for clinical analysis because it requires no exosome extraction, RNA purification, reverse transcription, or amplification and instead requires only a small volume of plasma (only 20 μL of plasma is needed for exosomal miRNA analysis) and can be performed in approximately 30 minutes with 1 pM sensitivity [70].
While blood is commonly obtained in liquid biopsy, clinicians may request urinary samples for certain types of cancer, such as bladder cancer [71] and prostate cancer [72]. Urinary exosomes have been shown to be promising biomarkers of renal-associated pathologies [73]. Increased α1-integrin and β1-integrin levels were found in urinary exosomes from metastatic prostate cancer patients compared to those from nonmetastatic patients [74]. In addition to its use in combination with tetraspanins (CD63, CD81 and CD9) as a general exosome marker, EpCAM is a specific urine exosomal marker of carcinoma cells that can be captured by antibody-coated magnetic microbeads [75]. Campos-Silva and coworkers demonstrated that only 500 μL of urine is required to isolate sufficient exosomes for detecting EpCAM expression by flow cytometry [75]. This study described a sensitive and reproducible method for the effective immunocapture of exosomes for future clinical applications. Finally, to address the challenge of the low abundance of urinary miRNAs, Wang and colleagues optimized a number of parameters for droplet digital PCR to achieve much higher sensitivity, reproducibility and accuracy than real-time quantitative PCR [76]. The exosome-based liquid biopsy techniques presented in this section are summarized in Table 1.
Superiority of aptamers as targeting ligands
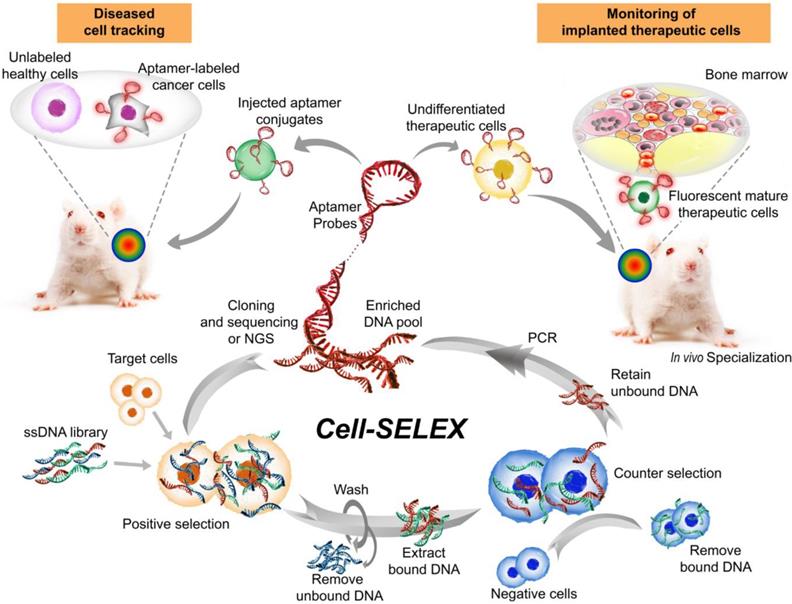
Aptamers, also known as chemical antibodies, are single-stranded DNA or RNA that fold into 3D structures and specifically bind to their targets with high affinity and specificity [80]. Aptamers are generated via a PCR-based in vitro selection strategy known as systematic evolution of ligands by exponential enrichment (SELEX) [81, 82] (Fig. 3). Owing to their advantages over conventional antibodies in terms of low immunogenicity and batch-to-batch variation, high specificity and sensitivity, as well as deeper tumor penetration [83, 84], aptamers have become an invaluable class of affinity ligands in biomedical research, diagnostics [85] and therapeutics [86]. For example, a DNA aptamer was developed by Wang and colleagues with superb targeting properties and distinctive functional versatility for early disease detection, imaging, and targeted delivery of therapeutic agents [87]. Liu et al. developed a cell-specific DNA aptamer-based fluorescence probe for the molecular subtyping of breast cancer [88]. This aptamer probe can distinguish, within 30 minutes, not only between different breast cancer cells but also between breast cancer cells and normal mammary epithelial cells, and between different tumors in mouse xenograft models of human breast cancer [88]. Such a robust aptamer has great potential to be further developed into a rapid and sensitive tool for guiding personalized therapy and determining prognosis [89, 90].
Aptamers are emerging as a new class of ligands for tracking tumors at the cellular, subcellular and molecular levels. Aptamers used in super-resolution microscopy offer many advantages over conventional antibody-based immunostaining methods, particularly in cases in which the affinity ligands themselves are larger than the protein of interest. Specifically, aptamer-based probes have improved detection sensitivity due to minimized steric hindrance, increased ligand density and high penetration into cells/tissues [91]. Indeed, studies from independent laboratories confirmed that the use of aptamers as detection ligands resulted in superior resolutions well below the light diffraction limit compared to the use of antibodies as probes [91-93]. Moreover, to achieve better resolution of aptamer-based probes, Spiegelmer technology has been used to select stable aptamer-based probes that achieve the best imaging quality. In this strategy, endonuclease-sensitive RNA aptamers (D-form) are replaced with endonuclease-resistant RNA aptamers (L-form) or mirror-image aptamers [91, 94].
Major steps involved in one cycle of cell-SELEX to isolate aptamer probes for cell tracking. Briefly, after the incubation of a random single-stranded aptamer library with target cells and subsequent washing steps, negative selection is performed to remove nonspecific binding sequences. Subsequently, the target-bound sequences are PCR-amplified prior to being subjected to the next cycle. The aptamers can then be attached to therapeutic cells ex vivo to track their biodistribution or differentiation in stem cell therapy following implantation into the host. Alternatively, the labeled aptamers can be used for specifically monitoring target cells. Images of the bone marrow and mice were modified from https://commons.wikimedia.org/wiki/File:Gray72.png by Henry Vandyke Carter and from https://www.stockvault.net/photo/126860/white-mouse by 2happy, respectively, with permissions.
Typical and most recent techniques of EV-based liquid biopsy with potential applications to cancer diagnosis.
| Technique | Biomarker | Cancer | Volume of samples | Time | Limit of detection | Reference |
|---|---|---|---|---|---|---|
| ExoDxTM Prostate (The first exosome-based liquid biopsy test approved by the FDA) | ERG, PCA3, and SPDEF (internal reference) | Prostate | ~ 20 mL (urine) | NA | prostate-specific antigen 2-10 ng mL-1; SPDEF detected at >30 copies per reaction | [61, 77] |
| ExoDxTM Lung (ALK) | EML4-ALK | Lung | 0.9-1.5 mL (plasma) | NA | 2.5 copies per reaction | [78, 79] |
| ExoTEST | CD63, Rab5b, and caveolin-1 | Melanoma | 100 µL (plasma) | ~ 3 days | less than 50 pg of targeted exosomal protein | [39] |
| ExoScreen | CD9, CD63, CD147, CEA, and CA19-9 | Colorectal | 5 µL (serum) | ~ 120 min | NA | [63] |
| Nanoplasmonic assay (nPLEX) | CD24, CD41, CD45, CD63, CA125, CA19-9, D2-40, EpCAM, EGFR, HER2, CLDN3, and MUC18 | Ovarian | NA (ascites) | ~ 30 min | ~ 3000 exosomes | [13] |
| EV array | ~ 60 biomarkers, including tetraspanin markers, EpCAM, NY-ESO-1, MUC16, CEA, CD151, CD142, CD146, EGFR, PDL-1, MET, and p53, CD13 | Non-small cell lung carcinoma | 1-10 µL (plasma) | ~ 3 days | 2.5 × 104 per microarray spot (~1 nL) | [66] |
| Microfluidic chip (self-assembled 3D herringbone nanoporous structure) | CD24, EpCAM, and folate receptor alpha proteins | Ovarian | 2 µL (plasma) | ~ 90 min | 10 exosomes μL-1 | [67] |
| Nickel-based isolation (NBI)-alpha or digital PCR | PSMA, KRAS, and BRAF | Prostate, colorectal | 20 µL (plasma) | ~180 min | NA | [68] |
| ExoProfile chip (3D porous serpentine nanostructures) | EGFR, HER2, CA125, FRα, CD24, EpCAM, CD9, and CD63 | Ovarian | 10 µL (plasma) | ~180 min | 21 exosomes μL-1 | [69] |
| Surface acoustic wave (SAW)-based microfluidic chip | miR-21, miR-550, and miR-146a | Liver, pancreatic, oral | 20 µL (plasma) | ~ 30 min | 1 pM (miRNA concentration) | [70] |
| Antibody-coated magnetic microbeads followed by flow cytometry | CD63, CD81, CD9, and EpCAM | Prostate | 500 µL (urine) | ~ 2 days | 30 ng of exosomes (1.37 × 107 particles) | [75] |
| Droplet digital PCR | miR-29a | Healthy volunteers | 0.3-2 µL (urine) | NA | 5 copies μL-1 | [76] |
The in vivo targeting of aptamers to tumor cells can be visualized by labeling with a suitable fluorescent agent or radionuclide. 18F-radiolabeled HER2-targeted DNA aptamers were developed and demonstrated high tumor uptake. Therefore, such aptamers hold promise as specific HER2-positive tumor imaging agents in positron emission tomography (PET) [95, 96]. In addition, FAM and Cy5 were used to label the R13 aptamer to investigate the mechanism of aptamer internalization and the ability to target ovarian cancer cells [97]. A bright, orange fluorescent turn-on probe (TMR-DN) bound to a rainbow aptamer, SRB-2, was used to improve the signal-to-background ratio in fluorescence imaging, providing low background fluorescence and enabling no-wash live-cell RNA imaging [98]. This novel aptamer-based system was used to image the distinct subcellular localization patterns of ribosomal RNA and mRNA in bacteria and mammalian cells [98]. A recent study showed that Cy5-labeled M17, a DNA aptamer that specifically recognizes MMP14, is a promising molecular probe for imaging numerous types of MMP14-positive cancer cells [99]. Moreover, the past 10 years have witnessed the development of quantum dot (QD)-labeled aptamers and their extensive applications in cancer theranostics [100, 101]. For example, a versatile, sensitive and selective sandwich assay involving a DNA aptamer and QDs as signal amplifiers was established for cancer detection [102]. Additionally, EGFR aptamer-conjugated lipid nanoparticles containing QDs and siRNAs were used to achieve remarkable EGFR-dependent siRNA delivery and fluorescence imaging [103].
Simple yet powerful aptamer sensors can be developed on a DNA module platform composed of an aptamer, a joint module for sensing a conformational change in the aptamer, a terminal stem, and a DNAzyme to report target detection in a concentration-dependent manner. Using such a strategy, Tomita and co-workers customized a microarray containing more than 10,000 sequences designed by in silico secondary structure predictions for array-based screening [104]. Aptamer-based biosensors have demonstrated practical application in point-of-care drug monitoring systems, whereby the circulating drug concentration can be detected in human serum [105]. For the design of such a point-of-care device, the biointerface of the sensor may consist of a binary self-assembled monolayer of a specific thiolated aptamer and 6-mercapto-1-hexanol (MCH, a surface blocking agent and aptamer spacer) at an optimized ratio based on electrochemical impedance spectroscopy measurements, which are used to enhance the sensitivity towards specific targets [106]. Memristive nanowires, assembled based on the regeneration properties of DNA aptamers on their surface, have also emerged as potential real-time monitoring tools for ultrasensitive, highly specific and selective drug biosensors. Such systems have been used for the successful detection of tenofovir in human serum [107]. Electrochemical aptamer-based sensors constitute another type of promising biosensor for the rapid, specific recognition and quantification of drugs such as insulin. In this device, a redox label-modified guanine-rich aptamer that folds into a G-quadruplex serves as as a probe to detect insulin, enabling researchers to discriminate insulin, glucagon and somatostatin in a Krebs-Ringer bicarbonate buffer [108]. Another sandwich-type electrochemical aptamer-based biosensor, known as an aptasensor, was designed with a combination of tetrahedral DNA aptamers and a flower-like nanozyme/horseradish peroxidase (HRP) combination and used for the detection of the breast cancer cell biomarker HER2 [109]. Specifically, in the assembly of this biosensor, the aptamer specifically binds to HER2 and the complex is immobilized on the gold electrode surface, where the Mn3O4- Pd@Pt nanozymes are linked by another aptamer and natural enzyme HRP, collectively referred to as nanoprobe 1, to amplify the biosensor signal. To further amplify the signal, nanoprobe 1 is subsequently converted into dendritic DNA nanostructures via links with nanoprobe 2, a structure based on Pd@Pt/HRP/cDNA [109].
Since the development of aptamer technology nearly 30 years ago, pegaptanib, an RNA aptamer against vascular endothelial growth factor (VEGF), is the only aptamer that has been approved by the FDA for the treatment of macular degeneration [110]. Nevertheless, a number of aptamers are undergoing preclinical and clinical development, including aptamers to thrombin, nucleolin and prostate-specific membrane antigen [111]. Three strategies have been commonly used for the therapeutic application of aptamers, namely, (i) aptamers as antagonists, (ii) aptamers as agonists, and (iii) aptamers as delivery agents [112]. All the aptamers in current clinical trials have been classified as category (i) aptamers, and the design of most must be improved to meet the expectations for therapeutic efficacy, such as the generation of a hybrid complex with an antibody [113], conjugation with cholesterol [114] or nanoparticles [115], or formulation as a multimer [116]. On the other hand, a few aptamers have been developed as agonists, and they include RNA aptamers against HER3/ERBB3, OX40 (CD134), 4-1BB (CD137), CD40 and CD28 and DNA aptamers targeting human VEGFR-2 and insulin receptor [112]. As targeting ligands in drug delivery systems, aptamers function to guide the systems to the desired cells/tissues to maximize treatment efficacy and minimize systemic toxicity [117]. By neutralizing histones with chemically stabilized anionic 2′ fluoro-modified RNA aptamers with high binding affinity, Giangrande's group demonstrated the efficacy of histone-specific aptamers in the treatment of multiple clinical conditions associated with multiple organ dysfunction syndrome [117]. One new tool generated by aptamer-based techniques for nanomedicine precision therapy is a DNA nanorobot. Ma et al. generated a new intelligent DNA nanorobot based on an anti-HER2 aptamer anchored to a tetrahedral nucleic acid framework for the selective lysosomal degradation of HER2 protein in breast cancer cells [118].
Although aptamers are promising ligands for targeted theranostics, one potential barrier for their translation into the clinic is the possible loss of targeting capacity under in vivo conditions. After performing studies to mitigate this concern, Tan's group recently reported the causes of this loss of targeting [119]. By determining variations in the surface chemistry and biological behavior of nanoparticles in serum, Tan's group identified several factors that contribute to the loss of efficacy of aptamer-guided nanocarriers. The rapid clearance of nanoparticles due to the immune response to the nanoparticle surface, aggregation of small nanoparticles, protein corona blocking, and enzymatic cleavage of the aptamer [119] are among the key mechanisms underlying the loss of aptamer-based targeting efficacy in vivo. Future smart design and engineering are expected to provide effective solutions to this problem.
Aptamer-guided exosome diagnostics and therapeutics
The application of aptamer technology in both basic science research and the clinical translation of exosomes is poised to lead to the development of the next generation of diagnostics and therapeutics.
Aptamer-guided exosome diagnostics
To exploit molecular markers on the surface of exosomes for improving exosome enrichment efficiency and facilitating exosome isolation, aptamers can be selected to specifically bind to the markers and thus capture the exosomes. The engineering of aptamer-guided exosome diagnostic tools is outlined in the following sections.
Simple use of peptide/RNA/DNA aptamers to capture exosomes
By selecting an aptamer against an overexpressed protein on exosomes, an aptamer-guided exosome-capturing nanoplatform system can be developed as a diagnostic tool. Garrido and colleagues developed an aptamer against the A8 peptide that could bind to the extracellular domain of HSP70, which is overexpressed in many cancers [120]. This aptamer was utilized to capture HSP70-positive exosomes in urine samples from patients with breast, lung, or ovarian cancer [121]. This study demonstrates a key advantage of quantifying cancer-derived exosomes over determining the number of circulating tumor cells: there are more exosomes than cancer cells in systemic circulation. Moreover, exosomes can be quantified in both blood and urine. Murakami et al. selected two RNA aptamers of 55 and 30 nucleotides (nt) after several rounds of SELEX [122]. Both the 55- and 30-nt aptamers had strong affinity for exosomes, as analyzed by SPR, whereas circular dichroism spectroscopy revealed that the two aptamers could form a G-quadruplex structure in their loop regions that was stabilized by potassium ions [122]. In a separate work, Sun's group developed λ-DNA- and DNA aptamer-mediated approaches for the simultaneous size-selective separation and surface protein analysis of exosomes [123]. Impressively, a machine learning algorithm applied to exosomal size and marker signatures was able to distinguish various breast cell lines and stage II breast cancer patients with varied HER2 expression patterns [123].
Aptasensor
Given their advantages of high sensitivity, rapid response, portability, and low sample volume requirement, aptasensors have become an important diagnostic tool for detecting cancer-derived exosomes [124] (Fig. 4).
Wang et al. presented a nanotetrahedron (NTH)-assisted electrochemical aptasensor as a sensitive and rapid tool for the direct capture and detection of exosomes secreted by hepatocellular carcinoma cells [125]. In this study, an aptamer containing one expanded nucleotide was generated against liver cancer cells, and it exhibited selectivity for exosomes secreted from HepG2 cells. The NTH-assisted aptasensors had improved specificity and capture efficiency. This study provides a strategy for overcoming the limitations of aptasensors that impede exosome detection, including the likelihood that immobilized aptamers on electrodes undergo self-assembled monolayer aggregation or entanglement and the inability to control the spatial orientation of single-stranded aptamers [125]. Another technical challenge relates to the efficient detection of cancer-derived exosomes among a background of exosomes secreted by non-cancerous cells in order to evaluate the diagnostic results or to gain a comprehensive understanding of the tumor migration and/or invasion mechanism. To overcome these challenges, Huang et al. developed a label‐free electrochemical aptasensor containing an anti‐CD63 antibody-modified gold electrode and an aptamer against mucin. The mucin aptamer was linked to a primer sequence complementary to a G‐quadruplex circular template and was used to detect gastric cancer exosomes [126]. The high sensitivity of this method was based on the effects of the hemin/G-quadruplex DNAzyme on H2O2 reduction to influence the production of electrochemical signals as well as a signal amplification step. This is achieved through rolling circle amplification, a reaction that generates high molecular weight products to accomplish signal amplification [126]. Such an electrochemical aptasensor has a detection limit of ~ 100 cancer biomarker-positive exosomes per milliliter.
A scheme of exosome isolation and/or analysis via an electrochemical aptasensor. In this platform, the electrode is made of gold and carbon surfaces for the detection of different target analytes, e.g., tumor-derived exosomes of various sizes. Changes in the redox signal are proportional to the concentration of exosomes. After aptamer immobilization followed by incubation with exosomes, the signal is typically significantly suppressed due to the decreased electrode surface area. The image of the potentiostat was modified from https://mep.metrohm.com.au/2017/07/29/metrohm-autolab-portable-potentiostat-kit/ with permission.
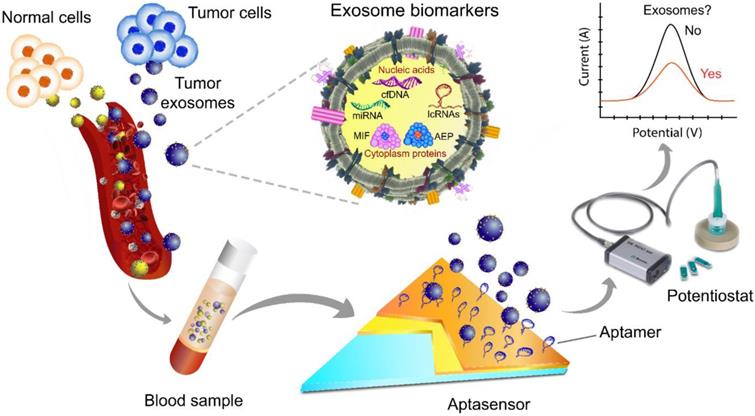
Other lab-on-a-chip-based diagnostic tools
Most aptamer-guided exosome diagnostic tools are based on the principle of selective binding between aptamers and exosome/disease markers. Inorganic materials and polymers are common components used in conjunction with aptamers to form integrated platforms for lab-on-a-chip exosomal diagnostic devices. Zhang and colleagues developed the ExoAPP assay, an aptamer nanoprobe-based exosome profiling system that can be used to phenotype exosome surface proteins and quantify cancer-derived exosomes [127]. This tool consists of a graphene oxide interfacing with aptamers against exosome markers. The profiling of exosome markers is achieved by the integration of enzyme-assisted exosome recycling. This assay can detect exosomes at the limit of 1.6 × 105 particles/ mL. The ability of ExoAPP to identify surface PSMA on target exosomes in blood samples from prostate cancer patients is indicative of its future application in clinical diagnostics [127]. Furthermore, to enrich EpCAM-positive cancer-derived exosomes for clinical detection, Yoshida et al. developed an EpCAM‐affinity coating agent (EpiVeta) to be used on the surface of inorganic materials in diagnostic devices [128]. EpiVeta consists of a conjugate of a peptide aptamer for EpCAM and an MPC polymer. The aptamer enables the versatility of the conjugation process, while the zwitterionic membrane‐mimicking polymer MPC consists of methacrylate with a phosphoryl‐choline polar group and is used to reduce the non‐specific binding of proteins to material surfaces [128]. In addition, Xu et al. reported a two-stage microfluidic platform termed the ExoPCD chip for EV on-chip isolation and in situ electrochemical analysis of exosomes from human serum using DNA aptamer sensors [129]. The ExoPCD chip utilized magnetic enrichment based on the specific recognition of the phosphatidylserine-Tim4 protein combined with a novel signal transduction pathway to detect CD63-positive exosomes with high sensitivity using only 30 µL of serum. Samples from liver cancer patients and healthy controls were distinguished by this chip, demonstrating that it is a promising tool for exosome analysis and non-invasive diagnostics [129]. Additionally, in developing a diagnostic tool based on the recognition of the surface protein CD63, Wang et al. fabricated a device consisting of three surface- enhanced Raman scattering (SERS) probes coupled with CD63 aptamer-based magnetic substrates for screening and detecting a broad range of exosomes [130]. Importantly, to simultaneously detect multiple types of exosomes, the gold nanoparticle probe was modified with different Raman reporters, each with a specific aptamer targeting the exosomes of interest. This system demonstrates great potential for further clinical applications in cancer screening because it can successfully detect target exosomes in blood samples from patients with breast cancer, colorectal cancer or prostate cancer [130].
Challenges to be addressed
Numerous new platforms have been developed to address the technical challenges in conventional methodologies regarding separation/isolation/c apture of exosomes. Researchers at the Chinese Academy of Sciences have developed an aptamer- based fluorescence polarization assay with a one-step mix-and-read platform for exosome quantification that requires less than 1 µL of plasma and has no purification or amplification step [131]. This technique, named the AFPExo assay, was developed to overcome the problem of exosome loss during conventional purification/isolation steps, such as ultracentrifugation, ultrafiltration and size exclusion chromatography. This assay is based on the principle that the molecular mass of dye-labeled aptamers is significantly altered after exosome binding, causing a significant change in the fluorescence polarization signal as analyzed with a plate reader. Furthermore, such signals are amplified by the inherent large mass/volume of exosomes. This assay can analyze as many as 5 × 105 exosomes in 1 μL within 30 minutes with a detection limit of 500 exosomes per microliter [131].
Furthermore, it is often desirable or necessary to release the captured exosomes from aptamers immobilized on a solid matrix. This can be achieved by the use of chelating agents, such as EDTA, which removes magnesium ions and disrupts the 3D structure of the aptamer, resulting in the release of the bound target. Unfortunately, such a chelating agent-based strategy may not work with all aptamers. Ideally, an affinity-based isolation technique should deliver intact exosomes that retain structural integrity and biological function for further downstream applications. To this end, Zhang and colleagues designed a CD63 aptamer- and magnetic bead-based approach that can be used to rapidly isolate exosomes and subsequently release intact captured exosomes within 90 minutes through the use of a DNA structure-switch aptamer [132]. The functional competence of the exosomes released after capture was confirmed with a wound healing assay [132].
Aptamer-guided exosome therapeutics
While the technologies for aptamer-based exosome diagnostics have been developed extensively, relatively few studies that have evaluated aptamer-targeted exosome delivery systems have been reported. Wan et al. reported aptamer-targeted exosomes carrying paclitaxel, an anticancer drug commonly included in chemotherapy regimens, as a new platform for clinical implementation in cancer treatment [133]. In this study, the nucleolin-targeting aptamer AS1411 was covalently conjugated to cholesterol-PEG and subsequently grafted onto mouse dendritic cell membranes. These cells were then mechanically extruded to create aptamer-guided exosomes. With this extrusion-based strategy, approximately 3 × 1010 aptamer-guided exosomes with a peak size of 105 nm were obtained from ~1 × 107 cells within one hour. Cholesterol-PEG2000 was chosen because of its amphiphilicity and relative rigidity to stabilize exosomes through hydrophobic- hydrophobic interactions at the lipid bilayer. The strategy for synthesizing the cholesterol-PEG2000- aptamers could potentially be utilized for large-scale production of targeted exosomes secreted from immune cells for the treatment of different cancers. This methodology was proposed to be potentially safer than cell-based immunotherapy (e.g., CAR T-cell therapy) because the cells used to prepare the exosomes can be obtained from the patients who need the therapy. Such patient-derived exosomes can be used for drug loading without the need for genetic engineering [133]. In an alternative approach, the orientation of the aptamer ligand on the surface of the exosomes was used to reprogram exosomes to control siRNA/miRNA loading and surface ligand display on exosomes with an RNA aptamer, which resulted in efficient targeted exosome delivery and cancer suppression [134]. Specifically, these authors engineered an RNA nanostructure with a three-way junction to control the ligand display on the surface of the EVs. The placement of membrane-anchoring cholesterol at the tail of the three-way arrow resulted in the display of the RNA aptamer or folate on the outer surface of the vesicles. In contrast, the placement of cholesterol at the arrowhead of the three-way arrow resulted in partial loading of RNA nanoparticles into the vesicles. As a result, the RNA nanostructure was directionally linked to the lipid bilayer membrane of the EVs, and the targeting ligands decorated the outer surface of the EVs. Such orientation-engineered ligand-displaying EVs could specifically deliver siRNA to cells and achieve an efficient blockade of tumor growth in the three cancer models studied [134]. Most recently, the aptamer sgc8, which can specifically recognizes membrane-bound protein tyrosine kinase 7 (PTK7), was conjugated to a diacyllipid via a PEG linker in a targeted anticancer therapeutic delivery platform [135]. The exosomes secreted by immature dendritic cells were loaded with doxorubicin by electroporation, followed by the surface functionalization of vesicles with the targeting ligand via the hydrophobic interaction between the diacyllipid tail of the aptamer conjugates and the phospholipid bilayer of the exosomes [135]. Such engineered sgc8-guided exosomes showed selective and dose-dependent cytotoxicity to human leukaemia (CEM) cells. Regarding the cellular uptake mechanism, studies showed that among multiple endocytosis pathways, clathrin-mediated endocytosis played a major role in sgc8 aptamer-mediated cellular internalization. These results indicate that the targeting ligands themselves may affect the interaction of exosomes with cells [135]. However, whether other aptamer-target pairs also affect exosome uptake by various types of cancer cells remains to be ascertained. Figure 5 illustrates a typical therapeutic application of aptamer-guided EVs in vivo.
Commentary on aptamer-guided exosome theranostics
The capture of exosomes using aptamers against exosomal markers such as tetraspanins and other cancer-specific markers, followed by the quantitative assessment of a disease-specific marker, constitutes an invaluable strategy for detecting disease at an early stage, monitoring the treatment response and making accurate prognostic predictions. The fact that there are more circulating exosomes in the peripheral blood of tumor patients than in that of healthy subjects suggests that tumor-derived exosomes are a potentially useful noninvasive diagnostic tool. The commonly used techniques to detect and quantify individual exosomes include nanoparticle tracking analysis (NTA) and flow cytometry. While the results obtained from NTA are subject to interference from contaminating lipoproteins and protein aggregates, which are similar to exosomes in size and density, most flow cytometers are unable to detect vesicles smaller than 100 nm. On the other hand, the specific detection of exosomal surface proteins enables an effective determination of exosomes. However, ELISA requires a relatively large number of exosomes with a detection limit of approximately 107 particles/ μL. Fortunately, fluorescence and electrochemical platforms with specifically designed aptamers can be highly sensitive. Compared with antibodies, aptamers have superior performance because they are much smaller. For instance, an EpCAM aptamer was shown to have better tumor penetration, more homogeneous distribution and better retention in vivo than an EpCAM antibody in a mouse xenograft tumor model [84]. Indeed, antibodies have poor access to intracellular targets and are more immunogenic and thermally unstable [136]. Other limitations of antibodies have been extensively reported, including the high production costs, unexpected pharmacokinetic profiles, unclear modes of action, poor tissue penetration and heterogeneous distribution [137, 138]. In contrast, aptamers have high affinity for the targeted exosomal membrane proteins [139]. Moreover, exosomes have a molecular mass of approximately 3.3 × 107 kDa [140], and aptamers are much smaller than antibodies (6~10 kDa vs. 150 kDa) [141]. An IgG molecule occupies an area of ~77 nm2, while a 25-nt aptamer covers an area of ~ 10 nm2. If both the antibody and 25-nt aptamer were conjugated to a single fluorophore or HRP, then the exosome surface area covered by one antibody would be equal to that of multiple aptamers if all other parameters were similar (e.g., equilibrium dissociation constant, on-rate and off-rate). Therefore, the use of aptamers as binding ligands in lieu of using antibodies will allow the more robust isolation and detection of exosomes. In addition, nucleic acid chemistry enables the large-scale and economical synthesis of aptamers with minimum batch-to-batch variations. Due to these distinct advantages, aptamers are becoming a highly attractive class of targeting ligands for engineering exosomes in theranostic applications (summarized in Table 2).
A typical manufacturing cycle of the EV-based engineering of exosomes or microvesicles for targeted therapeutics. In allogeneic EV therapy, EV-producing cells are isolated from individuals who are not genetically identical and then manipulated in vitro to load the therapeutic cargo. Subsequently, aptamers are decorated on the exosome or microvesicle surface to generate aptamer-targeted exosomes or microvesicles that are ready to deliver therapeutic cargos in a targeted system. In autologous EV therapy, the EV-producing cells are obtained from the patient requiring treatment, as EVs can be readily isolated from body fluids or produced by cultured mesenchymal stem cells from the patient, and then transferred back to the same patient after in vitro cargo loading and surface functionalization with aptamers. Images of the silhouette (mouse and girl) were downloaded for free from https://www.nicepng.com/ourpic/u2q8y3a9a9i1a9y3_mouse-silhouette-sidewasy-mouse-clip-art-silhouette-mouse/, and https://www.freepik.com/free-vector/black-girl-silhouettes_764970.htm#page=1&query=girl%20silhouette&position=1, designed by Freepik.
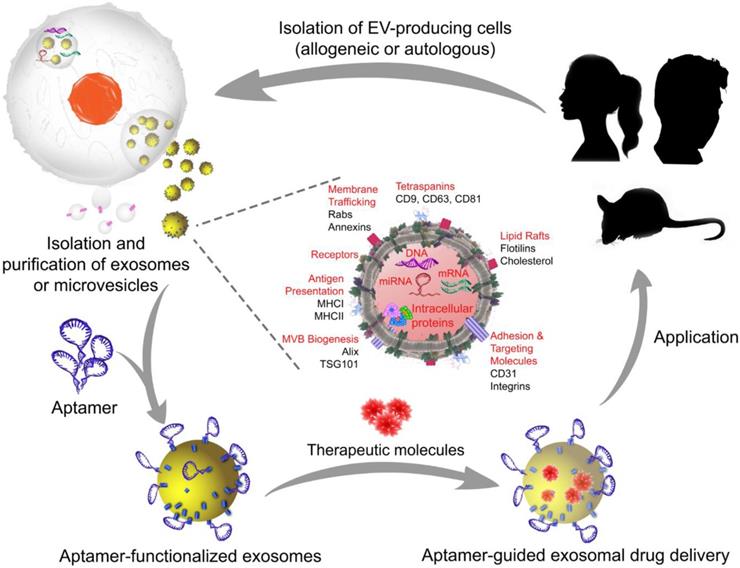
Prospects for next-generation theranostics
Since its humble beginnings 30 years ago, aptamer technology is now paving the way for the flourishing development of new and effective diagnostic and therapeutic tools for major diseases [142-146]. Taking advantage of their small size compared to antibodies and their exquisite binding affinity and specificity [112], aptamers can bind to some otherwise inaccessible targets, thereby increasing tissue penetration and conferring enhanced therapeutic efficacy [147, 148]. Chemical engineering and nanotechnological efforts have led to the generation of chemically modified aptamers with improved pharmacokinetic profiles in vivo [114]. Moreover, with their advantages of cost-efficiency, time-saving, minimal batch-to-batch variation and with the ability to release intact exosomes after purification, aptamers have become robust tools for both basic research and clinical translation in exosome-based targeted theranostics [112, 121]. Furthermore, aptamers against disease-specific exosomes can be selected using modified SELEX techniques such as counter-SELEX to enhance the specificity [149], thereby empowering targeted exosome therapeutics. At the diagnostic front, a number of advanced diagnostic tools for exosome isolation and/or detection have emerged to provide unprecedented sensitivity and specificity, such as nPLEX [13], the fluorescence-based microfluidic chips [150], the alternating current electrokinetic microarray chip [151], and interferometric imaging [152]. Future work will focus on expanding the development of assays for accurate, reproducible, sensitive, and rapid EV detection without a requirement for separation and washing steps. Eventually, clinically validated assays must be adapted for direct implementation in modern automated pathology laboratories. Ideally, some of these next-generation diagnostics will be adapted as hand-held devices for point-of-care use.
A key challenge in exosome research and development is exosome heterogeneity because each exosome is unique in its molecular and biochemical composition [153]. Currently, there is an acute lack of robust methods for isolating or purifying exosomes and microvesicles for both diagnostic and therapeutic applications. Although most published work in the field of EV research has focused on exosomes, hence the exosome-centric coverage in this review, microvesicles have emerged as key players in mediating intercellular communication between cancerous and stromal cells and in orchestrating complex pathophysiological processes [154-159]. Therefore, the next decade will witness renewed efforts in the development of microvesicle-based theranostics. Indeed, recent seminal work by Coffey and colleagues, for the first time, has established CD81/CD9/CD63 and annexin A1 as specific biomarkers for exosomes and microvesicles, respectively [7]. The future development of aptamers against these newly identified EV markers will greatly enhance our ability to effectively and efficiently isolate EV subpopulations. We will then be able to elucidate the mechanisms underlying EV biogenesis and heterogeneity and translate the results of EV research to pathology laboratories and the clinic.
Abbreviations
Aβ: β-amyloid; CA125: cancer antigen 125; cDNA: complementary DNA; EGFRs: epidermal growth factor receptors; ELISA: enzyme-linked immunosorbent assay; EpCAM: epithelial cell adhesion molecule; EPI: ExoDxTM Prostate IntelliScore; ESCRT: endosomal sorting complex required for transport; EV: extracellular vesicles; FDA: Food and Drug Administration; FRα: folate receptor α; HER2: human epidermal growth factor receptor 2; HRP: horseradish peroxidase; HSPs: heat shock proteins; ICAM-1: intercellular adhesion molecule-1; L1CAM: neural cell adhesion molecule L1; LFA-1: lymphocyte function-associated antigen 1; MCH: 6-mercapto-1-hexanol; mRNA: messenger RNA; miRNA: microRNA; MHC: major histocompatibility complex; MMP: metalloproteinase; MSC: mesenchymal stromal cell; MUC-1: Mucin-1; NfL: neurofilament light; nPLEX: nanoplasmonic exosome; nt: nucleotide; NTA: nanoparticle tracking analysis; NTH: nanotetrahedron; PCR: polymerase chain reaction; PD-L1: programmed death-ligand 1; PET: positron emission tomography; PLAP: placental alkaline phosphatase; PSMA: prostate-specific membrane antigen; PTK7: protein tyrosine kinase 7; QD: quantum dot; SELEX: systematic evolution of ligands by exponential enrichment; SERS: surface-enhanced Raman scattering; SPR: surface plasmon resonance; siRNA: small interfering RNA; TMR-DN: tetramethylrhodamine-dinitroaniline; Tsg101: tumor- susceptibility gene 101; TYRP2: tyrosinase-related protein-2; VEGF: vascular endothelial growth factor.
Summary of aptamers used in aptamer-guided exosome theranostics.
| Aptamer | Cells producing exosomes (protein target) | Clinical samples/ potential applications | Reference | |
|---|---|---|---|---|
| Diagnostics | A8 | HCT116, SW480, PC3, HeLa (HSP70) | Urine from patients with breast, lung, or ovarian cancer | [121] |
| MO-1, MO-2 | 293T, HeLa S3 (tetraspanin) | Cervical cancer | [122] | |
| H2 and SYL3C | BT-474 and SK-BR-3 (HER2 and EpCAM) | Breast cancer | [123] | |
| LZH8 | HepG2 | Hepatocarcinoma | [125] | |
| MUC_3 | SGC7901 (Mucin 1) | Plasma from gastric cancer patients | [126] | |
| EpCAM/ Ep114 | HT-29, HCT-15, and MCF-7 (EpCAM) | Colorectal, and breast cancer | [127, 128] | |
| CD63 | HepG2, A549, and MCF-7 | Serum from patients with gastric cancer, plasma from patients with non-small lung cancer or breast cancer | [127, 129, 131, 132] | |
| H2, CEA, and PSMA | SKBR3 (HER2), T84 (carcinoembryonic antigen), and LNCaP (PSMA) | Blood from patients with breast cancer, colorectal cancer, or prostate cancer | [130] | |
| Therapeutics | AS1411 | Dendritic cells (nucleolin) | Breast cancer | [133] |
| PSMA and EGFR | HEK293T | Breast, prostate, and colorectal cancer | [134] | |
| sgc8 | Dendritic cells (PTK7) | Leukemia/ Lymphoma | [135] |
Acknowledgements
Dr. Phuong Ha-Lien Tran is the recipient of the Australian Research Council's Discovery Early Career Researcher Award (project number DE160100900). We thank BioRad, Metrohm, and PerkinElmer for their permission to use their images and content.
Competing Interests
Wei Duan has received ongoing research funding from Suzhou GenePharma Co., Ltd.
References
1. Tkach M, Kowal J, Théry C. Why the need and how to approach the functional diversity of extracellular vesicles. Philos Trans R Soc Lond B Biol Sci. 2018;373:20160479
2. Colombo M, Raposo G, Théry C. Biogenesis, Secretion, and Intercellular Interactions of Exosomes and Other Extracellular Vesicles. Annu Rev Cell Dev Bi. 2014;30:255-89
3. Zijlstra A, Di Vizio D. Size matters in nanoscale communication. Nat Cell Biol. 2018;20:228-30
4. Wolf P. The Nature and Significance of Platelet Products in Human Plasma. Br J Haematol. 1967;13:269-88
5. Soares RP, Xander P, Costa AO, Marcilla A, Menezes-Neto A, Del Portillo H. et al. Highlights of the São Paulo ISEV workshop on extracellular vesicles in cross-kingdom communication. J Extracell Vesicles. 2017;6:1407213
6. Zhang H, Freitas D, Kim HS, Fabijanic K, Li Z, Chen H. et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat Cell Biol. 2018;20:332-43
7. Jeppesen DK, Fenix AM, Franklin JL, Higginbotham JN, Zhang Q, Zimmerman LJ. et al. Reassessment of Exosome Composition. Cell. 2019;177:428-445.e18
8. Zhang Y, Liu Y, Liu H, Tang WH. Exosomes: biogenesis, biologic function and clinical potential. Cell Biosci. 2019;9:19
9. Rodrigues M, Fan J, Lyon C, Wan M, Hu Y. Role of Extracellular Vesicles in Viral and Bacterial Infections: Pathogenesis, Diagnostics, and Therapeutics. Theranostics. 2018;8:2709-21
10. Mulcahy LA, Pink RC, Carter DRF. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles. 2014;3:10.3402 /jev.v3.24641
11. Yáñez-Mó M, Siljander PRM, Andreu Z, Zavec AB, Borràs FE, Buzas EI. et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:10.3402 /jev.v4.27066
12. Graner MW, Alzate O, Dechkovskaia AM, Keene JD, Sampson JH, Mitchell DA. et al. Proteomic and immunologic analyses of brain tumor exosomes. FASEB J. 2009;23:1541-57
13. Im H, Shao H, Park YI, Peterson VM, Castro CM, Weissleder R. et al. Label-free detection and molecular profiling of exosomes with a nano-plasmonic sensor. Nat Biotechnol. 2014;32:490-5
14. Shao H, Im H, Castro CM, Breakefield X, Weissleder R, Lee H. New Technologies for Analysis of Extracellular Vesicles. Chem Rev. 2018;118:1917-50
15. Skotland T, Sandvig K, Llorente A. Lipids in exosomes: Current knowledge and the way forward. Prog Lipid Res. 2017;66:30-41
16. Jiang L, Gu Y, Du Y, Liu J. Exosomes: Diagnostic Biomarkers and Therapeutic Delivery Vehicles for Cancer. Mol Pharm. 2019;16:3333-49
17. Chen G, Huang AC, Zhang W, Zhang G, Wu M, Xu W. et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. 2018;560:382-6
18. Hurwitz NS, Meckes GD. Extracellular Vesicle Integrins Distinguish Unique Cancers. Proteomes. 2019;7:14
19. Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL. et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci U S A. 2008;105:10513-8
20. Cheng L, Sharples RA, Scicluna BJ, Hill AF. Exosomes provide a protective and enriched source of miRNA for biomarker profiling compared to intracellular and cell-free blood. J Extracell Vesicles. 2014;3:10.3402 /jev.v3.23743
21. Ferrante SC, Nadler EP, Pillai DK, Hubal MJ, Wang Z, Wang JM. et al. Adipocyte-derived exosomal miRNAs: a novel mechanism for obesity-related disease. Pediatr Res. 2015;77:447-54
22. Ghai V, Wu X, Bheda-Malge A, Argyropoulos CP, Bernardo JF, Orchard T. et al. Genome-wide Profiling of Urinary Extracellular Vesicle microRNAs Associated With Diabetic Nephropathy in Type 1 Diabetes. Kidney Int Rep. 2017;3:555-72
23. Solé C, Cortés-Hernández J, Felip ML, Vidal M, Ordi-Ros J. miR-29c in urinary exosomes as predictor of early renal fibrosis in lupus nephritis. Nephrol Dial Transpl. 2015;30:1488-96
24. Yang VK, Loughran KA, Meola DM, Juhr CM, Thane KE, Davis AM. et al. Circulating exosome microRNA associated with heart failure secondary to myxomatous mitral valve disease in a naturally occurring canine model. J Extracell Vesicles. 2017;6:1350088
25. Chen J, Hu C, Pan P. Extracellular Vesicle MicroRNA Transfer in Lung Diseases. Front Physiol. 2017;8:1028
26. Jan AT, Malik MA, Rahman S, Yeo HR, Lee EJ, Abdullah TS. et al. Perspective Insights of Exosomes in Neurodegenerative Diseases: A Critical Appraisal. Front Aging Neurosci. 2017;9:317
27. Sun B, Dalvi P, Abadjian L, Tang N, Pulliam L. Blood neuron-derived exosomes as biomarkers of cognitive impairment in HIV. AIDS. 2017;31:F9-F17
28. Tarasoff-Conway JM, Carare RO, Osorio RS, Glodzik L, Butler T, Fieremans E. et al. Clearance systems in the brain-implications for Alzheimer disease. Nat Rev Neurol. 2015;11:457-70
29. Mitsuhashi S, Feldbrügge L, Csizmadia E, Mitsuhashi M, Robson SC, Moss AC. Luminal Extracellular Vesicles (EVs) in Inflammatory Bowel Disease (IBD) Exhibit Proinflammatory Effects on Epithelial Cells and Macrophages. Inflamm Bowel Dis. 2016;22:1587-95
30. Tannetta D, Collett G, Vatish M, Redman C, Sargent I. Syncytiotrophoblast extracellular vesicles - Circulating biopsies reflecting placental health. Placenta. 2017;52:134-8
31. Salomon C, Torres MJ, Kobayashi M, Scholz-Romero K, Sobrevia L, Dobierzewska A. et al. A gestational profile of placental exosomes in maternal plasma and their effects on endothelial cell migration. PLOS ONE. 2014;9:e98667
32. Sarker S, Scholz-Romero K, Perez A, Illanes SE, Mitchell MD, Rice GE. et al. Placenta-derived exosomes continuously increase in maternal circulation over the first trimester of pregnancy. J Transl Med. 2014;12:204
33. Aqrawi LA, Galtung HK, Vestad B, Øvstebø R, Thiede B, Rusthen S. et al. Identification of potential saliva and tear biomarkers in primary Sjögren's syndrome, utilising the extraction of extracellular vesicles and proteomics analysis. Arthritis Res Ther. 2017;19:14
34. Tran PHL, Wang T, Yin W, Tran TTD, Barua HT, Zhang Y. et al. Development of a nanoamorphous exosomal delivery system as an effective biological platform for improved encapsulation of hydrophobic drugs. Int J Pharm. 2019;566:697-707
35. Zhang ZG, Buller B, Chopp M. Exosomes — beyond stem cells for restorative therapy in stroke and neurological injury. Nat Rev Neurol. 2019;15:193-203
36. Conway A-M, Mitchell C, Kilgour E, Brady G, Dive C, Cook N. Molecular characterisation and liquid biomarkers in Carcinoma of Unknown Primary (CUP): taking the 'U' out of 'CUP'. Br J Cancer. 2019;120:141-53
37. Kosaka N, Kogure A, Yamamoto T, Urabe F, Usuba W, Prieto-Vila M. et al. Exploiting the message from cancer: the diagnostic value of extracellular vesicles for clinical applications. Exp Mol Med. 2019;51:31
38. Miller AM, Shah RH, Pentsova EI, Pourmaleki M, Briggs S, Distefano N. et al. Tracking tumour evolution in glioma through liquid biopsies of cerebrospinal fluid. Nature. 2019;565:654-8
39. Logozzi M, De Milito A, Lugini L, Borghi M, Calabrò L, Spada M. et al. High levels of exosomes expressing CD63 and caveolin-1 in plasma of melanoma patients. PLOS ONE. 2009;4:e5219
40. König L, Kasimir-Bauer S, Bittner A-K, Hoffmann O, Wagner B, Santos Manvailer LF. et al. Elevated levels of extracellular vesicles are associated with therapy failure and disease progression in breast cancer patients undergoing neoadjuvant chemotherapy. Oncoimmunology. 2017;7:e1376153
41. Kosaka N, Yoshioka Y, Fujita Y, Ochiya T. Versatile roles of extracellular vesicles in cancer. J Clin Invest. 2016;126:1163-72
42. Melo SA, Luecke LB, Kahlert C, Fernandez AF, Gammon ST, Kaye J. et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature. 2015;523:177-82
43. Frampton AE, Prado MM, López-Jiménez E, Fajardo-Puerta AB, Jawad ZAR, Lawton P. et al. Glypican-1 is enriched in circulating-exosomes in pancreatic cancer and correlates with tumor burden. Oncotarget. 2018;9:19006-13
44. Peinado H, Alečković M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G. et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18:883-91
45. Keller S, König A-K, Marmé F, Runz S, Wolterink S, Koensgen D. et al. Systemic presence and tumor-growth promoting effect of ovarian carcinoma released exosomes. Cancer Lett. 2009;278:73-81
46. Keller L, Werner S, Pantel K. Biology and clinical relevance of EpCAM. Cell Stress. 2019;3:165-80
47. Massoner P, Thomm T, Mack B, Untergasser G, Martowicz A, Bobowski K. et al. EpCAM is overexpressed in local and metastatic prostate cancer, suppressed by chemotherapy and modulated by MET-associated miRNA-200c/205. Br J cancer. 2014;111:955-64
48. Fang S, Tian H, Li X, Jin D, Li X, Kong J. et al. Clinical application of a microfluidic chip for immunocapture and quantification of circulating exosomes to assist breast cancer diagnosis and molecular classification. PLOS ONE. 2017;12:e0175050
49. Julich-Haertel H, Urban SK, Krawczyk M, Willms A, Jankowski K, Patkowski W. et al. Cancer-associated circulating large extracellular vesicles in cholangiocarcinoma and hepatocellular carcinoma. J Hepatol. 2017;67:282-92
50. Kabe Y, Suematsu M, Sakamoto S, Hirai M, Koike I, Hishiki T. et al. Development of a Highly Sensitive Device for Counting the Number of Disease-Specific Exosomes in Human Sera. Clin Chem. 2018;64:1463-73
51. Roca E, Lacroix R, Judicone C, Laroumagne S, Robert S, Cointe S. et al. Detection of EpCAM-positive microparticles in pleural fluid: A new approach to mini-invasively identify patients with malignant pleural effusions. Oncotarget. 2016;7:3357-66
52. Roca E, Lacroix R, Judicone C, Laroumagne S, Robert S, Cointe S. et al. C Detection of EpCAM-positive microparticles in pleural fluid: A new approach for the diagnosis of the tumoral origin of pleural effusions. Eur Respir J. 2015;46:OA5005
53. Taylor DD, Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecol Oncol. 2008;110:13-21
54. Rabinowits G, Gerçel-Taylor C, Day JM, Taylor DD, Kloecker GH. Exosomal MicroRNA: A Diagnostic Marker for Lung Cancer. Clin Lung Cancer. 2009;10:42-6
55. Ostenfeld MS, Jensen SG, Jeppesen DK, Christensen L-L, Thorsen SB, Stenvang J. et al. miRNA profiling of circulating EpCAM(+) extracellular vesicles: promising biomarkers of colorectal cancer. J Extracell Vesicles. 2016;5:31488
56. Sánchez CA, Andahur EI, Valenzuela R, Castellón EA, Fullá JA, Ramos CG. et al. Exosomes from bulk and stem cells from human prostate cancer have a differential microRNA content that contributes cooperatively over local and pre-metastatic niche. Oncotarget. 2015;7:3993-4008
57. Jin X, Chen Y, Chen H, Fei S, Chen D, Cai X. et al. Evaluation of Tumor-Derived Exosomal miRNA as Potential Diagnostic Biomarkers for Early-Stage Non-Small Cell Lung Cancer Using Next-Generation Sequencing. Clin Cancer Res. 2017;23:5311-9
58. Rodríguez-Martínez A, de Miguel-Pérez D, Ortega FG, García-Puche JL, Robles-Fernández I, Exposito J. et al. Exosomal miRNA profile as complementary tool in the diagnostic and prediction of treatment response in localized breast cancer under neoadjuvant chemotherapy. Breast Cancer Res. 2019;21:21
59. Administrator ER. World's First Exosomal RNA-Based Liquid Biopsy is Launched. Exosome RNA. 2016
60. Sathianathen Niranjan J, Kuntz Karen M, Alarid-Escudero F, Lawrentschuk Nathan L, Bolton Damien M, Murphy Declan G. et al. Incorporating Biomarkers into the Primary Prostate Biopsy Setting: A Cost-Effectiveness Analysis. J Urol. 2018;200:1215-20
61. McKiernan J, Donovan MJ, Margolis E, Partin A, Carter B, Brown G. et al. A Prospective Adaptive Utility Trial to Validate Performance of a Novel Urine Exosome Gene Expression Assay to Predict High-grade Prostate Cancer in Patients with Prostate-specific Antigen 2-10ng/ml at Initial Biopsy. Eur Urol. 2018;74:731-8
62. Properzi F, Logozzi M, Fais S. Exosomes: the future of biomarkers in medicine. Biomark Med. 2013;7:769-78
63. Yoshioka Y, Kosaka N, Konishi Y, Ohta H, Okamoto H, Sonoda H. et al. Ultra-sensitive liquid biopsy of circulating extracellular vesicles using ExoScreen. Nat Commun. 2014;5:3591
64. Vlassov AV, Magdaleno S, Setterquist R, Conrad R. Exosomes: Current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta. 2012;1820:940-8
65. Jørgensen M, Bæk R, Pedersen S, Søndergaard EKL, Kristensen SR, Varming K. Extracellular Vesicle (EV) Array: microarray capturing of exosomes and other extracellular vesicles for multiplexed phenotyping. J Extracell Vesicles. 2013;2:10.3402 /jev.v2i0.20920
66. Jakobsen KR, Paulsen BS, Bæk R, Varming K, Sorensen BS, Jørgensen MM. Exosomal proteins as potential diagnostic markers in advanced non-small cell lung carcinoma. J Extracell Vesicles. 2015;4:26659
67. Zhang P, Zhou X, He M, Shang Y, Tetlow AL, Godwin AK. et al. Ultrasensitive detection of circulating exosomes with a 3D-nanopatterned microfluidic chip. Nat Biomed Eng. 2019;3:438-51
68. Notarangelo M, Zucal C, Modelska A, Pesce I, Scarduelli G, Potrich C. et al. Ultrasensitive detection of cancer biomarkers by nickel-based isolation of polydisperse extracellular vesicles from blood. EBioMedicine. 2019;43:114-26
69. Zhang P, Zhou X, Zeng Y. Multiplexed immunophenotyping of circulating exosomes on nano-engineered ExoProfile chip towards early diagnosis of cancer. Chem Sci. 2019;10:5495-5504
70. Ramshani Z, Zhang C, Richards K, Chen L, Xu G, Stiles BL. et al. Extracellular vesicle microRNA quantification from plasma using an integrated microfluidic device. Commun Biol. 2019;2:189
71. Khadjavi A, Mannu F, Destefanis P, Sacerdote C, Battaglia A, Allasia M. et al. Early diagnosis of bladder cancer through the detection of urinary tyrosine-phosphorylated proteins. Br J Cancer. 2015;113:469-75
72. Guo J, Yang J, Zhang X, Feng X, Zhang H, Chen L. et al. A Panel of Biomarkers for Diagnosis of Prostate Cancer Using Urine Samples. Anticancer Res. 2018;38:1471-7
73. Pomatto MAC, Gai C, Bussolati B, Camussi G. Extracellular Vesicles in Renal Pathophysiology. Front Mol Biosci. 2017;4:37
74. Bijnsdorp IV, Geldof AA, Lavaei M, Piersma SR, van Moorselaar RJA, Jimenez CR. Exosomal ITGA3 interferes with non-cancerous prostate cell functions and is increased in urine exosomes of metastatic prostate cancer patients. J Extracell Vesicles. 2013;2:10.3402 /jev.v2i0.22097
75. Campos-Silva C, Suárez H, Jara-Acevedo R, Linares-Espinós E, Martinez-Piñeiro L, Yáñez-Mó M. et al. High sensitivity detection of extracellular vesicles immune-captured from urine by conventional flow cytometry. Sci Rep. 2019;9:2042
76. Wang C, Ding Q, Plant P, Basheer M, Yang C, Tawedrous E. et al. Droplet digital PCR improves urinary exosomal miRNA detection compared to real-time PCR. Clin Biochem. 2019;67:54-9
77. Donovan MJ, Noerholm M, Bentink S, Belzer S, Skog J, O'Neill V. et al. A molecular signature of PCA3 and ERG exosomal RNA from non-DRE urine is predictive of initial prostate biopsy result. Prostate Cancer Prostatic Dis. 2015;18:370-5
78. Brinkmann K EJ, Tannous B, Hurley J, Castellanos-Rizaldos E, Enderle D, Koestler T, Spiel A, Mueller R, Brock G, O'Neill V, Skog J, Noerholm M. Exosomal RNA-based liquid biopsy detection of EML4-ALK in plasma from NSCLC patients. Presented at: 16th World Conference on Lung Cancer, September 6-9. 2015 Denver, CO, USA
79. Brinkmann K ED, Koestler T, Bentink S, Emenegger J, Spiel A, Mueller R, O'Neill V, Skog J, Carbone D, Noerholm M. Plasma-based diagnostics for detection of EML4-ALK fusion transcripts in NSCLC patients. Presented at: American Association for Cancer Research Annual Meeting, April 18 -22. 2015 Philadelphia, PA, USA
80. Sun H, Tan W, Zu Y. Aptamers: versatile molecular recognition probes for cancer detection. Analyst. 2016;141:403-15
81. Ellington AD, Szostak JW. In vitro selection of RNA molecules that bind specific ligands. Nature. 1990;346:818-22
82. Tuerk C, Gold L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science. 1990;249:505
83. Shigdar S, Qian C, Lv L, Pu C, Li Y, Li L. et al. The Use of Sensitive Chemical Antibodies for Diagnosis: Detection of Low Levels of Epcam in Breast Cancer. PLOS ONE. 2013;8:e57613
84. Xiang D, Zheng C, Zhou S-F, Qiao S, Tran PH-L, Pu C. et al. Superior Performance of Aptamer in Tumor Penetration over Antibody: Implication of Aptamer-Based Theranostics in Solid Tumors. Theranostics. 2015;5:1083-97
85. Zheng Y, Zhao Y, Di Y, Xiu C, He L, Liao S. et al. DNA aptamers from whole-serum SELEX as new diagnostic agents against gastric cancer. RSC Advances. 2019;9:950-7
86. Menne J, Eulberg D, Beyer D, Baumann M, Saudek F, Valkusz Z. et al. C-C motif-ligand 2 inhibition with emapticap pegol (NOX-E36) in type 2 diabetic patients with albuminuria. Nephrol Dial Transplant. 2017;32:307-15
87. Wang J, Gao T, Luo Y, Wang Z, Zhang Y, Zhang Y. et al. In Vitro Selection of a DNA Aptamer by Cell-SELEX as a Molecular Probe for Cervical Cancer Recognition and Imaging. J Mol Evol. 2019;87:72-82
88. Liu M, Wang Z, Tan T, Chen Z, Mou X, Yu X. et al. An Aptamer-Based Probe for Molecular Subtyping of Breast Cancer. Theranostics. 2018;8:5772-83
89. Sun H, Zu Y. A Highlight of Recent Advances in Aptamer Technology and Its Application. Molecules. 2015;20:11959-80
90. Yang S, Li H, Xu L, Deng Z, Han W, Liu Y. et al. Oligonucleotide Aptamer-Mediated Precision Therapy of Hematological Malignancies. Mol Ther Nucleic Acids. 2018;13:164-75
91. Ali HM, Elsherbiny EM, Emara M. Updates on Aptamer Research. Int J Mol Sci. 2019;20:2511
92. Gomes de Castro MA, Höbartner C, Opazo F. Aptamers provide superior stainings of cellular receptors studied under super-resolution microscopy. PLOS ONE. 2017;12:e0173050
93. de Castro MAG, Rammner B, Opazo F. Aptamer Stainings for Super-resolution Microscopy. In: (ed.) Mayer G. Nucleic Acid Aptamers: Selection, Characterization, and Application. New York, NY: Springer New York. 2016 p. 197-210
94. Oberthür D, Achenbach J, Gabdulkhakov A, Buchner K, Maasch C, Falke S. et al. Crystal structure of a mirror-image L-RNA aptamer (Spiegelmer) in complex with the natural L-protein target CCL2. Nat Commun. 2015;6:6923
95. Zhu G, Zhang H, Jacobson O, Wang Z, Chen H, Yang X. et al. Combinatorial Screening of DNA Aptamers for Molecular Imaging of HER2 in Cancer. Bioconjug Chem. 2017;28:1068-75
96. Kim HJ, Park JY, Lee TS, Song IH, Cho YL, Chae JR. et al. PET imaging of HER2 expression with an 18F-fluoride labeled aptamer. PLOS ONE. 2019;14:e0211047
97. Li F, Wang Q, Zhang H, Deng T, Feng P, Hu B. et al. Characterization of a DNA Aptamer for Ovarian Cancer Clinical Tissue Recognition and in Vivo Imaging. Cell Physiol Biochem. 2018;51:2564-74
98. Sunbul M, Jäschke A. SRB-2: a promiscuous rainbow aptamer for live-cell RNA imaging. Nucleic Acids Res. 2018;46:e110
99. Huang X, Zhong J, Ren J, Wen D, Zhao W, Huan Y. A DNA aptamer recognizing MMP14 for in vivo and in vitro imaging identified by cell-SELEX. Oncol Lett. 2019;18:265-74
100. Wen L, Qiu L, Wu Y, Hu X, Zhang X. Aptamer-Modified Semiconductor Quantum Dots for Biosensing Applications. Sensors (Basel). 2017;17:1736
101. Moutsiopoulou A, Broyles D, Dikici E, Daunert S, Deo SK. Molecular Aptamer Beacons and Their Applications in Sensing, Imaging, and Diagnostics. Small. 2019;15:1902248
102. Hansen JA, Wang J, Kawde A-N, Xiang Y, Gothelf KV, Collins G. Quantum-Dot/Aptamer-Based Ultrasensitive Multi-Analyte Electrochemical Biosensor. J. Am. Chem. Soc. 2006;128:2228-9
103. Kim MW, Jeong HY, Kang SJ, Choi MJ, You YM, Im CS. et al. Cancer-targeted Nucleic Acid Delivery and Quantum Dot Imaging Using EGF Receptor Aptamer-conjugated Lipid Nanoparticles. Sci Rep. 2017;7:9474
104. Tomita Y, Morita Y, Suga H, Fujiwara D. DNA module platform for developing colorimetric aptamer sensors. BioTechniques. 2016;60:285-92
105. Ferguson BS, Hoggarth DA, Maliniak D, Ploense K, White RJ, Woodward N. et al. Real-time, aptamer-based tracking of circulating therapeutic agents in living animals. Sci Transl Med. 2013;5:213ra165
106. Aliakbarinodehi N, Jolly P, Bhalla N, Miodek A, De Micheli G, Estrela P. et al. Aptamer-based Field-Effect Biosensor for Tenofovir Detection. Sci Rep. 2017;7:44409
107. Tzouvadaki I, Aliakbarinodehi N, De Micheli G, Carrara S. The memristive effect as a novelty in drug monitoring. Nanoscale. 2017;9:9676-84
108. Wu Y, Midinov B, White RJ. Electrochemical Aptamer-Based Sensor for Real-Time Monitoring of Insulin. ACS Sensors. 2019;4:498-503
109. Ou D, Sun D, Lin X, Liang Z, Zhong Y, Chen Z. A dual-aptamer-based biosensor for specific detection of breast cancer biomarker HER2 via flower-like nanozymes and DNA nanostructures. J Mater Chem B. 2019;7:3661-9
110. Doggrell SA. Pegaptanib: the first antiangiogenic agent approved for neovascular macular degeneration. Expert Opin Pharmacother. 2005;6:1421-3
111. Nimjee SM, White RR, Becker RC, Sullenger BA. Aptamers as Therapeutics. Annu Rev Pharmacol Toxicol. 2017;57:61-79
112. Zhou J, Rossi J. Aptamers as targeted therapeutics: current potential and challenges. Nat Rev Drug Discov. 2017;16:181-202
113. Heo K, Min S-W, Sung HJ, Kim HG, Kim HJ, Kim YH. et al. An aptamer-antibody complex (oligobody) as a novel delivery platform for targeted cancer therapies. J Control Release. 2016;229:1-9
114. Lee CH, Lee S-H, Kim JH, Noh Y-H, Noh G-J, Lee S-W. Pharmacokinetics of a Cholesterol-conjugated Aptamer Against the Hepatitis C Virus (HCV) NS5B Protein. Mol Ther Nucleic Acids. 2015;4:e254
115. Chen L, Rashid F, Shah A, Awan HM, Wu M, Liu A. et al. The isolation of an RNA aptamer targeting to p53 protein with single amino acid mutation. Proc. Natl. Acad. Sci. U.S.A. 2015;112:10002-7
116. Soule EE, Bompiani KM, Woodruff RS, Sullenger BA. Targeting Two Coagulation Cascade Proteases with a Bivalent Aptamer Yields a Potent and Antidote-Controllable Anticoagulant. Nucleic Acid Ther. 2016;26:1-9
117. Urak KT, Blanco GN, Shubham S, Lin L-H, Dassie JP, Thiel WH. et al. RNA inhibitors of nuclear proteins responsible for multiple organ dysfunction syndrome. Nat Commun. 2019;10:116
118. Ma W, Zhan Y, Zhang Y, Shao X, Xie X, Mao C. et al. An Intelligent DNA Nanorobot with in Vitro Enhanced Protein Lysosomal Degradation of HER2. Nano Lett. 2019;19:4505-17
119. Ding D, Zhang Y, Sykes EA, Chen L, Chen Z, Tan W. The influence of physiological environment on the targeting effect of aptamer-guided gold nanoparticles. Nano Res. 2019;12:129-35
120. Rérole A-L, Gobbo J, De Thonel A, Schmitt E, Pais de Barros JP, Hammann A. et al. Peptides and Aptamers Targeting HSP70: A Novel Approach for Anticancer Chemotherapy. Cancer Res. 2011;71:484-95
121. Gobbo J, Marcion G, Cordonnier M, Dias AMM, Pernet N, Hammann A. et al. Restoring Anticancer Immune Response by Targeting Tumor-Derived Exosomes With a HSP70 Peptide Aptamer. J. Natl. Cancer Inst. 2015;108:djv330
122. Murakami K, Zhao J, Yamasaki K, Miyagishi M. Biochemical and structural features of extracellular vesicle-binding RNA aptamers. Biomed Rep. 2017;6:615-26
123. Liu C, Zhao J, Tian F, Chang J, Zhang W, Sun J. λ-DNA- and Aptamer-Mediated Sorting and Analysis of Extracellular Vesicles. J. Am. Chem. Soc. 2019;141:3817-21
124. Zhao L, Huang Y, Dong Y, Han X, Wang S, Liang X. Aptamers and Aptasensors for Highly Specific Recognition and Sensitive Detection of Marine Biotoxins: Recent Advances and Perspectives. Toxins (Basel). 2018;10:427
125. Wang S, Zhang L, Wan S, Cansiz S, Cui C, Liu Y. et al. Aptasensor with Expanded Nucleotide Using DNA Nanotetrahedra for Electrochemical Detection of Cancerous Exosomes. ACS Nano. 2017;11:3943-9
126. Huang R, He L, Xia Y, Xu H, Liu C, Xie H. et al. A Sensitive Aptasensor Based on a Hemin/G-Quadruplex-Assisted Signal Amplification Strategy for Electrochemical Detection of Gastric Cancer Exosomes. Small. 2019;15:1900735
127. Jin D, Yang F, Zhang Y, Liu L, Zhou Y, Wang F. et al. ExoAPP: Exosome-Oriented, Aptamer Nanoprobe-Enabled Surface Proteins Profiling and Detection. Anal Chem. 2018;90:14402-11
128. Yoshida M, Hibino K, Yamamoto S, Matsumura S, Yajima Y, Shiba K. Preferential capture of EpCAM-expressing extracellular vesicles on solid surfaces coated with an aptamer-conjugated zwitterionic polymer. Biotechnol Bioeng. 2018;115:536-44
129. Xu H, Liao C, Zuo P, Liu Z, Ye B-C. Magnetic-Based Microfluidic Device for On-Chip Isolation and Detection of Tumor-Derived Exosomes. Anal Chem. 2018;90:13451-8
130. Wang Z, Zong S, Wang Y, Li N, Li L, Lu J. et al. Screening and multiple detection of cancer exosomes using an SERS-based method. Nanoscale. 2018;10:9053-62
131. Zhang Z, Tang C, Zhao L, Xu L, Zhou W, Dong Z. et al. Aptamer-based fluorescence polarization assay for separation-free exosome quantification. Nanoscale. 2019;11:10106-13
132. Zhang K, Yue Y, Wu S, Liu W, Shi J, Zhang Z. Rapid Capture and Nondestructive Release of Extracellular Vesicles Using Aptamer-Based Magnetic Isolation. ACS Sensors. 2019;4:1245-51
133. Wan Y, Wang L, Zhu C, Zheng Q, Wang G, Tong J. et al. Aptamer-Conjugated Extracellular Nanovesicles for Targeted Drug Delivery. Cancer Res. 2018;78:798-808
134. Pi F, Binzel DW, Lee TJ, Li Z, Sun M, Rychahou P. et al. Nanoparticle orientation to control RNA loading and ligand display on extracellular vesicles for cancer regression. Nat Nanotechnol. 2018;13:82-9
135. Zou J, Shi M, Liu X, Jin C, Xing X, Qiu L. et al. Aptamer-Functionalized Exosomes: Elucidating the Cellular Uptake Mechanism and the Potential for Cancer-Targeted Chemotherapy. Anal Chem. 2019;91:2425-30
136. Qu J, Yu S, Zheng Y, Zheng Y, Yang H, Zhang J. Aptamer and its applications in neurodegenerative diseases. Cell Mol Life Sci. 2017;74:683-95
137. Chames P, Van Regenmortel M, Weiss E, Baty D. Therapeutic antibodies: successes, limitations and hopes for the future. Br J Pharmacol. 2009;157:220-33
138. Cruz E, Kayser V. Monoclonal antibody therapy of solid tumors: clinical limitations and novel strategies to enhance treatment efficacy. Biologics. 2019;13:33-51
139. Jiang Y, Shi M, Liu Y, Wan S, Cui C, Zhang L. et al. Aptamer/AuNP Biosensor for Colorimetric Profiling of Exosomal Proteins. Angew. Chem. Int. Ed. Engl. 2017;56:11916-20
140. Kesimer M, Scull M, Brighton B, DeMaria G, Burns K, O'Neal W. et al. Characterization of exosome-like vesicles released from human tracheobronchial ciliated epithelium: a possible role in innate defense. FASEB J. 2009;23:1858-68
141. Yoshida Y, Waga I, Horii K. Quantitative and sensitive protein detection strategies based on aptamers. P Proteonomics Clin Appl. 2012;6:574-80
142. Kim MW, Jeong HY, Kang SJ, Jeong IH, Choi MJ, You YM. et al. Anti-EGF Receptor Aptamer-Guided Co-Delivery of Anti-Cancer siRNAs and Quantum Dots for Theranostics of Triple-Negative Breast Cancer. Theranostics. 2019;9:837-52
143. Zhou J, Lazar D, Li H, Xia X, Satheesan S, Charlins P. et al. Receptor-targeted aptamer-siRNA conjugate-directed transcriptional regulation of HIV-1. Theranostics. 2018;8:1575-90
144. Kim DTH, Bao DT, Park H, Ngoc NM, Yeo SJ. Development of a novel peptide aptamer-based immunoassay to detect Zika virus in serum and urine. Theranostics. 2018;8:3629-42
145. Chan HN, Xu D, Ho SL, He D, Wong MS, Li HW. Highly sensitive quantification of Alzheimer's disease biomarkers by aptamer-assisted amplification. Theranostics. 2019;9:2939-49
146. Lai WY, Wang JW, Huang BT, Lin EP, Yang PC. A Novel TNF-α-Targeting Aptamer for TNF-α-Mediated Acute Lung Injury and Acute Liver Failure. Theranostics. 2019;9:1741-51
147. Xiang D, Shigdar S, Bean AG, Bruce M, Yang W, Mathesh M. et al. Transforming doxorubicin into a cancer stem cell killer via EpCAM aptamer-mediated delivery. Theranostics. 2017;7:4071-86
148. Wang T, Gantier MP, Xiang D, Bean AG, Bruce M, Zhou S-F. et al. EpCAM Aptamer-mediated Survivin Silencing Sensitized Cancer Stem Cells to Doxorubicin in a Breast Cancer Model. Theranostics. 2015;5:1456-72
149. Jenison RD, Gill SC, Pardi A, Polisky B. High-resolution molecular discrimination by RNA. Science. 1994;263:1425-9
150. Zhao Z, Yang Y, Zeng Y, He M. A microfluidic ExoSearch chip for multiplexed exosome detection towards blood-based ovarian cancer diagnosis. Lab Chip. 2016;16:489-96
151. Ibsen SD, Wright J, Lewis JM, Kim S, Ko S-Y, Ong J. et al. Rapid Isolation and Detection of Exosomes and Associated Biomarkers from Plasma. ACS Nano. 2017;11:6641-51
152. Daaboul GG, Gagni P, Benussi L, Bettotti P, Ciani M, Cretich M. et al. Digital Detection of Exosomes by Interferometric Imaging. Sci Rep. 2016;6:37246
153. Raposo G, Stahl PD. Extracellular vesicles: a new communication paradigm? Nat. Rev. Mol. Cell Biol. 2019;20:509-10
154. Bodega G, Alique M, Puebla L, Carracedo J, Ramírez RM. Microvesicles: ROS scavengers and ROS producers. J Extracell Vesicles. 2019;8:1626654
155. Guo Y, Tan J, Miao Y, Sun Z, Zhang Q. Effects of Microvesicles on Cell Apoptosis under Hypoxia. Oxid Med Cell Longev. 2019;2019:5972152
156. Stahl PD, Raposo G. Extracellular Vesicles: Exosomes and Microvesicles, Integrators of Homeostasis. Physiology. 2019;34:169-77
157. Bian X, Xiao Y-T, Wu T, Yao M, Du L, Ren S. et al. Microvesicles and chemokines in tumor microenvironment: mediators of intercellular communications in tumor progression. Mol Cancer. 2019;18:50
158. Agrahari V, Agrahari V, Burnouf P-A, Chew CH, Burnouf T. Extracellular Microvesicles as New Industrial Therapeutic Frontiers. Trends Biotechnol. 2019;37:707-29
159. Panfoli I, Santucci L, Bruschi M, Petretto A, Calzia D, Ramenghi LA. et al. Microvesicles as promising biological tools for diagnosis and therapy. Expert Rev Proteomic. 2018;15:801-8
Author contact
![]() Corresponding authors: Emails: Jiang_guoqincom and wei.duanedu.au
Corresponding authors: Emails: Jiang_guoqincom and wei.duanedu.au
 Global reach, higher impact
Global reach, higher impact