13.3
Impact Factor
Theranostics 2020; 10(8):3722-3736. doi:10.7150/thno.42008 This issue Cite
Research Paper
Stepwise targeting and responsive lipid-coated nanoparticles for enhanced tumor cell sensitivity and hepatocellular carcinoma therapy
1. Department of Pharmacy, School of Medicine, Shenzhen University, Shenzhen 518060, China
2. Shanghai Institute of Materia Medica, Chinese Academy of Sciences, 501 Haike Road, Shanghai 201203, China
3. School of Pharmacy, Shanghai University of Traditional Chinese Medicine, Shanghai 201203, China
Abstract
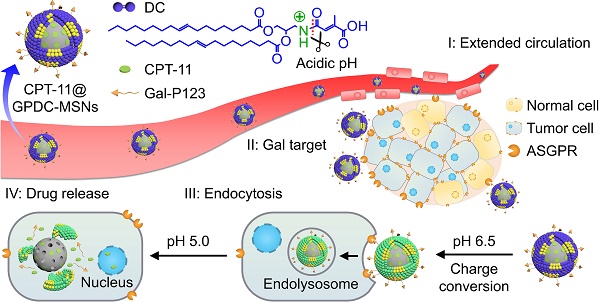
Rationale: Antitumor drug delivery faces multiple barriers that require consecutively achieving tumor targeting, selective cellular uptake and sufficient intracellular drug dosage.
Methods: Herein, we designed smart nanoparticles (GPDC-MSNs) that can accumulate stepwise in tumor tissues, selectively enter cancer cells by responding to the acidic tumor extracellular environment, and achieve considerable drug release in the intracellular microenvironment. The GPDC-MSNs comprise the synthesized material galactosyl-conjugated PEO-PPO-PEO (Gal-P123) for hepatocellular carcinoma (HCC) targeting, the tumor extracellular pH-responsive lipid (2E)-4-(dioleostearin)-amino-4-carbonyl-2-butenonic (DC) for selective cellular internalization, and antitumor drug irinotecan (CPT-11)-loaded mesoporous silica nanoparticles (MSNs) for on-demand intracellular drug release.
Results: GPDC-MSNs are negatively charged at pH 7.4 and promote active HCC targeting mediated by the asialoglycoprotein receptor. Upon reaching the weakly acidic tumor microenvironment, the nanoparticles undergo charge conversion to neutral, enhancing cellular internalization. Moreover, the encapsulated CPT-11 can be retained within GPDC-MSNs in the blood circulation but undergo intracellular burst release, which facilitates the apoptosis of tumor cells. GPDC-MSNs significantly increased HCC selectivity in vivo and exhibited up to 25 times higher accumulation in tumor tissue than in normal hepatic tissue, thus achieving superior antitumor efficacy and low systemic toxicity.
Conclusion: This stepwise-responsive nanoparticle should serve as a valuable platform and promising strategy for HCC treatment.
Keywords: drug delivery, pH sensitive, charge conversion, tumor targeting, hepatocellular carcinoma
 Global reach, higher impact
Global reach, higher impact