13.3
Impact Factor
Theranostics 2020; 10(8):3366-3381. doi:10.7150/thno.41849 This issue Cite
Research Paper
Targeting GRP78-dependent AR-V7 protein degradation overcomes castration-resistance in prostate cancer therapy
1. Affiliated Cancer Hospital & institute of Guangzhou Medical University, Protein Modification and Degradation Key Lab of Guangzhou and Guangdong, State Key Laboratory of Respiratory Disease, School of Basic Medical Sciences, Guangzhou Medical University, Guangzhou, Guangdong 510095, China
2. Department of Surgery, UT Southwestern Medical Center, Dallas, Texas 75390, USA
*Yuning Liao, Yuan Liu, and Xiaohong Xia contributed equally to this work.
Abstract
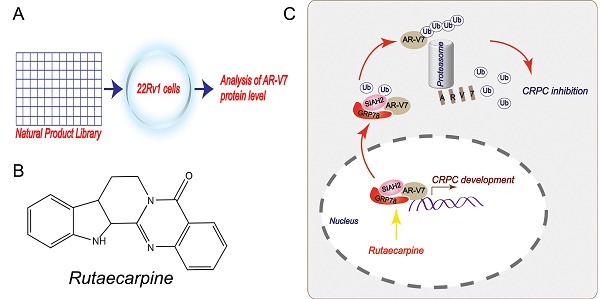
Rationale: Androgen receptor splice variant 7 (AR-V7) is a leading cause of the development of castration-resistant prostate cancer (CRPC). However, the regulation and function of AR-V7 at levels of post-translational modifications in prostate cancer therapy remain poorly understood. Here, we conducted a library screen of natural products to identify potential small molecules responsible for AR-V7 protein degradation in human prostate cancer cell lines.
Methods: A natural product library was used to screen the inhibitor of AR-V7. Co-IP and biomass spectrum assays were used to identify the AR-V7-interacting proteins, whereas western blot, confocal microscopy, RNA interfering, and gene transfection were used to validate these interactions. Cell viability, EDU staining, and colony formation assays were employed to detect cell growth and proliferation. Flowcytometry assays were used to detect the distribution of cell cycle. Mouse xenograft models were used to study the anti-CRPC effects in vivo.
Results: This screen identified rutaecarpine, one of the major components of the Chinese medicine Evodia rutaecarpa, as a novel chemical that selectively induces AR-V7 protein degradation via K48-linked ubiquitination. Mechanically, this effect relies on rutaecarpine inducing the formation of a GRP78-AR-V7 protein complex, which further recruits the E3 ligase SIAH2 to directly promote the ubiquitination of AR-V7. Consequently, the genetic and pharmacological activation of the GRP78-dependent AR-V7 protein degradation restores the sensitivity of castration-resistant prostate cancer to anti-androgen therapy in cell culture and animal models.
Conclusions: These findings not only provide a new approach for overcoming castration-resistance in prostate cancer therapy, but also increase our understanding about the interplay between molecular chaperones and ubiquitin ligase in shaping protein stability.
Keywords: AR-V7, CRPC, rutaecarpine, GRP78, SIAH2
 Global reach, higher impact
Global reach, higher impact