13.3
Impact Factor
Theranostics 2020; 10(4):1910-1922. doi:10.7150/thno.36936 This issue Cite
Research Paper
TGF-β inhibition combined with cytotoxic nanomedicine normalizes triple negative breast cancer microenvironment towards anti-tumor immunity
1. Cancer Biophysics Laboratory, Department of Mechanical and Manufacturing Engineering, University of Cyprus, Nicosia, Cyprus.
2. Department of Life Sciences, Program in Biological Sciences, European University Cyprus, Nicosia, Cyprus.
3. Department of Bioengineering, Graduate School of Engineering, The University of Tokyo, Bunkyo, Tokyo, Japan.
4. The Center for the Study of Haematological Malignancies, Nicosia, Cyprus.
5. Karaiskakio Foundation, Nicosia, Cyprus.
6. Innovation Center of NanoMedicine, Kawasaki Institute of Industrial Promotion, Kawasaki, Kanagawa, Japan.
7. Institute of Future Initiatives, The University of Tokyo, Bunkyo, Tokyo, Japan.
Abstract
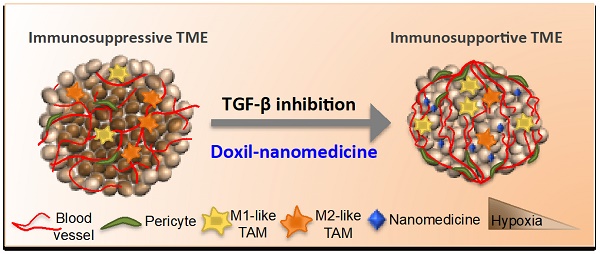
Tumor normalization strategies aim to improve tumor blood vessel functionality (i.e., perfusion) by reducing the hyper-permeability of tumor vessels or restoring compressed vessels. Despite progress in strategies to normalize the tumor microenvironment (TME), their combinatorial antitumor effects with nanomedicine and immunotherapy remain unexplored.
Methods: Here, we re-purposed the TGF-β inhibitor tranilast, an approved anti-fibrotic and antihistamine drug, and combined it with Doxil nanomedicine to normalize the TME, increase perfusion and oxygenation, and enhance anti-tumor immunity. Specifically, we employed two triple-negative breast cancer (TNBC) mouse models to primarily evaluate the therapeutic and normalization effects of tranilast combined with doxorubicin and Doxil. We demonstrated the optimized normalization effects of tranilast combined with Doxil and extended our analysis to investigate the effect of TME normalization to the efficacy of immune checkpoint inhibitors.
Results: Combination of tranilast with Doxil caused a pronounced reduction in extracellular matrix components and an increase in the intratumoral vessel diameter and pericyte coverage, indicators of TME normalization. These modifications resulted in a significant increase in tumor perfusion and oxygenation and enhanced treatment efficacy as indicated by the notable reduction in tumor size. Tranilast further normalized the immune TME by restoring the infiltration of T cells and increasing the fraction of T cells that migrate away from immunosuppressive cancer-associated fibroblasts. Furthermore, we found that combining tranilast with Doxil nanomedicine, significantly improved immunostimulatory M1 macrophage content in the tumorigenic tissue and improved the efficacy of the immune checkpoint blocking antibodies anti-PD-1/anti-CTLA-4.
Conclusion: Combinatorial treatment of tranilast with Doxil optimizes TME normalization, improves immunostimulation and enhances the efficacy of immunotherapy.
Keywords: tumor microenvironment, vascular perfusion, normalization, nanomedicine, immunostimulation, immunotherapy
 Global reach, higher impact
Global reach, higher impact