13.3
Impact Factor
Theranostics 2020; 10(3):1415-1432. doi:10.7150/thno.40857 This issue Cite
Research Paper
Denervation drives skeletal muscle atrophy and induces mitochondrial dysfunction, mitophagy and apoptosis via miR-142a-5p/MFN1 axis
1. Department of Hand Surgery, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430022, China.
2. Department of Pharmacy, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430030, China.
3. Department of Plastic and Hand Surgery, Technical University of Munich, Munich 81675, Germany.
Abstract
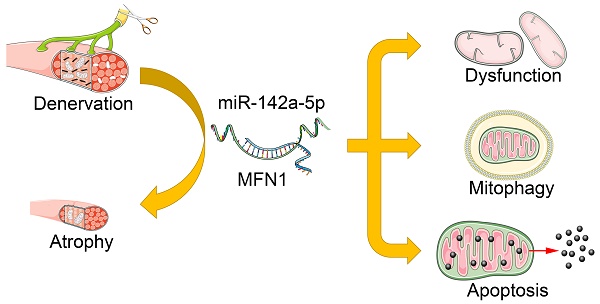
Rationale: Peripheral nerve injury is common in clinic, which leads to severe atrophy and dysfunction of the denervated muscles, but the underlying mechanism is not fully understood. Recent studies advanced the causative role of mitochondrial dysfunction in muscle atrophy, while the upstream triggers remained unclear.
Methods: In the present study, Atrophy of gastrocnemius and tibialis anterior (TA) were evaluated in mice sciatic nerve transection model. Transmission electron microscopy (TEM) was then used to observe the microstructure of atrophic gastrocnemius and mitochondria. Subsequently, small RNA sequencing, luciferase reporter assay and Electrophoretic Mobility Shift (EMSA) were performed to explore the potential signaling pathway involved in skeletal muscle atrophy. The effects of the corresponding pathway on mitochondrial function, mitophagy, apoptosis and muscle atrophy were further determined in C2C12 cells and denervated gastrocnemius.
Results: Gastrocnemius and TA atrophied rapidly after denervation. Obvious decrease of mitochondria number and activation of mitophagy was further observed in atrophic gastrocnemius. Further, miR-142a-5p/ mitofusin-1 (MFN1) axis was confirmed to be activated in denervated gastrocnemius, which disrupted the tubular mitochondrial network, and induced mitochondrial dysfunction, mitophagy and apoptosis. Furthermore, the atrophy of gastrocnemius induced by denervation was relieved through targeting miR-142a-5p/MFN1 axis.
Conclusions: Collectively, our data revealed that miR-142a-5p was able to function as an important regulator of denervation-induced skeletal muscle atrophy by inducing mitochondrial dysfunction, mitophagy, and apoptosis via targeting MFN1. Our findings provide new insights into the mechanism of skeletal muscle atrophy following denervation and propose a viable target for therapeutic intervention in individuals suffering from muscle atrophy after peripheral nerve injury.
Keywords: denervation, skeletal muscle atrophy, miRNA-142a-5p, MFN1, mitophagy, apoptosis
 Global reach, higher impact
Global reach, higher impact