13.3
Impact Factor
Theranostics 2020; 10(26):12158-12173. doi:10.7150/thno.52000 This issue Cite
Research Paper
In situ tumor-triggered subcellular precise delivery of multi-drugs for enhanced chemo-photothermal-starvation combination antitumor therapy
1. Medical School of Southeast University, Nanjing 210009, People's Republic of China.
2. Center of Clinical Laboratory Medicine, Zhongda Hospital, Southeast University, Nanjing 210009, People's Republic of China.
3. Jiangsu Provincial Key Laboratory of Critical Care Medicine, Southeast University, Nanjing 210009, People's Republic of China.
Abstract
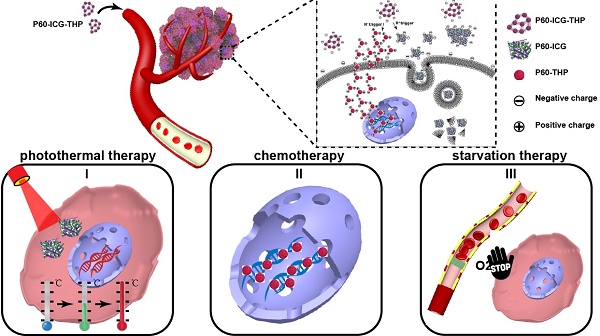
Rationale: Drug combination therapy for cancer treatment exerts a more potent antitumor effect. The targeted delivery and release of multiple drugs in a patient's body thus presents a more effective treatment approach, warranting further research. Methods: Two antitumor drugs (ICG: indocyanine green and THP: pirarubicin) were successfully screened to sequentially trigger self-assembling peptides (P60) to produce bacteria-sized particles (500-1000 nm, P60-ICG-THP). First, after mixing equal amount of P60 and ICG, trace amount of water (the mass ratio between P60 and water: 100:1) was used to trigger their assembly into P60-ICG. Subsequently, the assembly of P60-ICG and THP was further triggered by ultrasound treatment to produce P60-ICG-THP. Results: P60-ICG-THP constituted a cluster of several nanoparticles (50-100 nm) and possessed a negative charge. Owing to its size and charge characteristics, P60-ICG-THP could remain outside the cell membrane, avoiding the phagocytic clearance of blood and normal tissue cells in vivo. However, after localizing in the tumor, the size and charge switches of P60-ICG-THP, rapidly triggered by the low pH of the tumor microenvironment, caused P60-ICG-THP to segregate into two parts: (i) positively charged nanoparticles with a size of approximately 50 nm, and (ii) negatively charged particles of an uneven size. The former, mainly carrying THP (chemotherapeutic agent), could immediately cross the cell membrane and deliver pirarubicin into the nucleus of tumor cells. The latter, carrying ICG (used for photothermal therapy), could also enter the cell via the endocytosis pathway or accumulate in tumor blood vessels to selectively block the supply of nutrients and oxygen (cancer starvation). Both these particles could avoid the rapid excretion of ICG in the liver and were conducive to accumulation in the tumor tissue for photothermal therapy. Conclusion: Our drug delivery system not only achieves the precise subcellular delivery of two anticancer drugs due to their size and charge switches in the tumor site, but also provides a new strategy to combine chemotherapy, photothermal therapy, and cancer starvation therapy for the development of a highly efficient antitumor therapeutic regimen.
Keywords: drug combination therapy, self-assembling peptide, triggered drug release, subcellular drug delivery, size and charge switches.
 Global reach, higher impact
Global reach, higher impact