13.3
Impact Factor
Theranostics 2020; 10(25):11690-11706. doi:10.7150/thno.51316 This issue Cite
Research Paper
Concurrent visual and acoustic tracking of passive and active delivery of nanobubbles to tumors
1. Department of Medical Biophysics, University of Toronto, Toronto, Ontario, Canada.
2. Princess Margaret Cancer Research Centre, Toronto, Ontario, Canada.
3. Sunnybrook Research Institute, Toronto, Ontario, Canada.
4. Department of Radiology, Case Western Reserve University, Cleveland OH 44106, United States
Abstract
Background: There has been growing interest in nanobubbles for their potential to extend bubble-mediated ultrasound approaches beyond that of their larger microbubble counterparts. In particular, the smaller scale of nanobubbles may enable them to access the tumor extravascular compartment for imaging and therapy in closer proximity to cancer cells. Compelling preliminary demonstrations of the imaging and therapeutic abilities of nanobubbles have thus emerged, with emphasis on their ability to extravasate. However, studies to date rely on indirect histologic evidence that cannot confirm whether the structures remain intact beyond the vasculature - leaving their extravascular potential largely untapped.
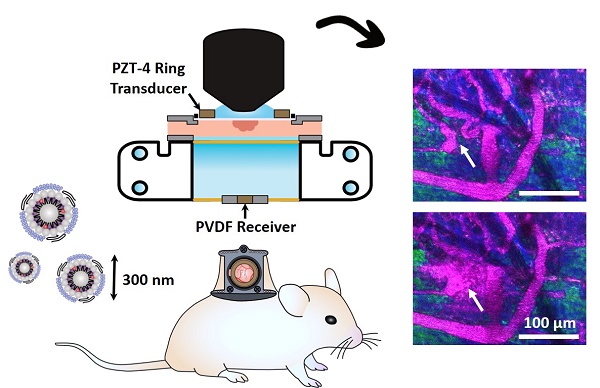
Methods: Nanobubble acoustic scattering was assessed using a recently reported ultra-stable formulation at low concentration (106 mL-1) and frequency (1 MHz), over a range of pressures (100-1500 kPa) in a channel phantom. The pressure-dependent response was utilized as a basis for in vivo experiments where ultrasound transmitters and receivers were integrated into a window chamber for simultaneous intravital multiphoton microscopy and acoustic monitoring in tumor-affected microcirculation. Microscopy and acoustic data were utilized to assess passive and active delivery of nanobubbles and determine whether they remained intact beyond the vasculature.
Results: Nanobubbles exhibit pressure-dependent nonlinear acoustic scattering. Nanobubbles are also found to have prolonged acoustic vascular pharmacokinetics, and passively extravasate intact into tumors. Ultrasound stimulation of nanobubbles is shown to actively enhance the delivery of both intact nanobubbles and shell material, increasing their spatial bioavailability deeper into the extravascular space. A range of acute vascular effects were also observed.
Conclusion: This study presents the first direct evidence that nanobubbles passively and actively extravasate intact in tumor tissue, and is the first to directly capture acute vascular events from ultrasound-stimulation of nanobubbles. The insights gained here demonstrate an important step towards unlocking the potential of nanobubbles and extending ultrasound-based applications.
Keywords: extravasation, intravital imaging, multiphoton microscopy, nanobubble, ultrasound
 Global reach, higher impact
Global reach, higher impact