13.3
Impact Factor
Theranostics 2020; 10(23):10573-10588. doi:10.7150/thno.48666 This issue Cite
Research Paper
Cadherin-11 cooperates with inflammatory factors to promote the migration and invasion of fibroblast-like synoviocytes in pigmented villonodular synovitis
1. Institute of Sports Medicine, Beijing Key Laboratory of Sports Injuries, Peking University Third Hospital, 49 North Garden Road, Haidian District, Beijing 100191, People's Republic of China.
2. State Key Laboratory of Membrane Biology, Institute of Molecular Medicine, Peking University, 5 Yiheyuan Road, Haidian District, Beijing 100871, People's Republic of China.
3. Institute of Molecular Medicine, Beijing Key Laboratory of Cardiometabolic Molecular Medicine, Peking University, 5 Yiheyuan Road, Haidian District, Beijing 100871, People's Republic of China.
#Contributed equally to this work.
Abstract
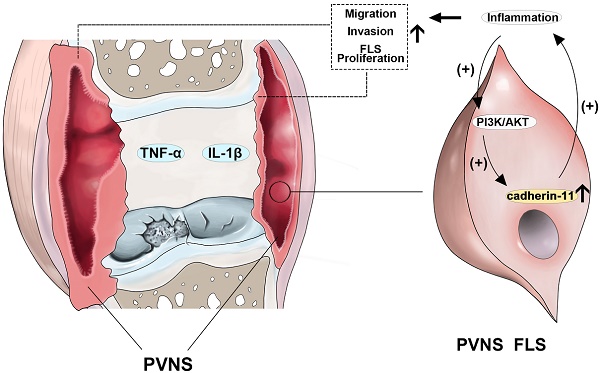
Rationale: Pigmented villonodular synovitis (PVNS) is a destructive benign tumor-like hyperplastic disease that occurs in synovial tissue. Fibroblast-like synoviocytes (FLS) are the predominant cell type comprising the structure of the PVNS synovial lining layer. Due to a high recurrence rate, high invasion, migration, and cartilage destruction ability, PVNS causes substantial damage to patients and the efficacy of surgical resection is not satisfactory. Therefore, exploring the pathogenesis and identifying novel therapeutic targets for PVNS are urgently required. Currently, the pathogenesis of PVNS remains unclear, and there is uncertainty and controversy regarding whether PVNS is an inflammatory or a neoplastic disease. Cadherin-11 is a classical molecule that mediates hemophilic cell-to-cell adhesion in FLS and plays an important role in the normal synovium lining layer formation. This study aimed to explore the role of inflammation and cadherin-11 in PVNS pathogenesis and determine the effects of cadherin-11 as a molecular target for PVNS treatment.
Methods: FLS were primarily cultured from PVNS patients during arthroscopic synovectomy. The level of cytokines in the PVNS synovial fluid was evaluated using a human antibody array. Cadherin-11 expression of PVNS FLS was detected by qPCR, Western blots, tissue immunohistochemistry, and cell immunofluorescence. Cadherin-11 was down-regulated by siRNA or up-regulated with a plasmid, with or without inflammatory factor stimulation, and PI3K/Akt was inhibited with LY294002. The capacity of migration and invasion of PVNS FLS was tested using Transwell and wound-healing assays. Activation of the nuclear factor-kappaB (NF-κB) and mitogen-activated protein kinase (MAPK) pathways was detected by Western blots. Chondrocyte damage by PVNS FLS was assessed with a co-culture assay.
Results: Inflammatory factors (IL-1β and TNF-α) in the synovial fluid of PVNS patients were significantly up-regulated. Cadherin-11 was highly expressed in the FLS of PVNS patients, and positively correlated with recurrence, extra-articular migration, and cartilage destruction of PVNS. Knocking down of cadherin-11 inhibited the migration and invasion of PVNS FLS. Moreover, inflammatory factors up-regulated the expression of cadherin-11, which activated the NF-κB and MAPK signaling pathways and led to cartilage destruction. Inhibition of cadherin-11 blocked IL-1β- and TNF-α-induced activation of the above pathways, migration and invasion of PVNS FLS, and damage of chondrocyte. In addition, the elevation of cadherin-11 expression, together with the migration and invasion, of PVNS FLS was down-regulated by the inhibition of the PI3K/Akt signaling pathway.
Conclusions: Cadherin-11 plays an important role in the pathogenesis of PVNS and forms a positive feedback loop with inflammatory factors, which further activates the NF-κB and MAPK pathways to trigger an inflammatory cascade. Cadherin-11-mediated inflammation results in PVNS with high recurrence, invasiveness, and strong cartilage destruction ability, and eventually promotes the transformation of PVNS from the initial inflammatory disease to neoplastic disease. Thus, inhibition of cadherin-11 together with its related inflammatory reaction, represents a new therapeutic strategy for PVNS.
Keywords: pigmented villonodular synovitis, cadherin-11, inflammation, fibroblast-like synoviocytes, targeted therapy
 Global reach, higher impact
Global reach, higher impact