13.3
Impact Factor
Theranostics 2020; 10(23):10563-10572. doi:10.7150/thno.48522 This issue Cite
Research Paper
Association of sex and APOE ε4 with brain tau deposition and atrophy in older adults with Alzheimer's disease
1. Department of Radiology, Xuanwu Hospital, Capital Medical University, Beijing, China.
2. Mallinckrodt Institute of Radiology, Washington University in St. Louis School of Medicine, St. Louis, MO, USA.
3. Harvard-MIT Program in Health Sciences and Technology, Harvard Medical School, Boston, MA, USA.
4. Department of Neurology, Washington in St. Louis University School of Medicine, St. Louis, MO, USA.
Received 2020-5-21; Accepted 2020-8-4; Published 2020-8-21
Abstract
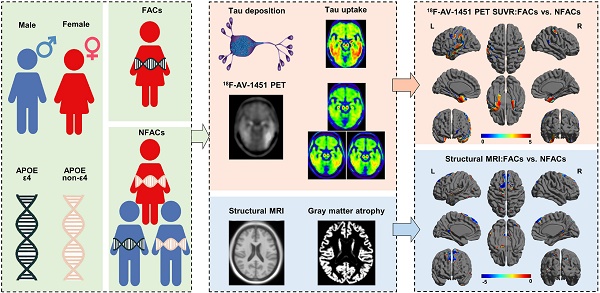
The objective of this study was to assess the association of sex and the apolipoprotein E (APOE) ε4 allele with brain tau deposition and atrophy in older adults with Alzheimer's disease (AD) using quantitative 18F-AV-1451 positron emission tomography (PET) and magnetic resonance imaging (MRI).
Methods: Preprocessed 18F-AV-1451 tau PET, raw T1-weighted structural MR images, demographic information, cerebrospinal fluid (CSF) total tau (t-tau) and phosphorylated tau (p-tau) measurements from 57 elderly individuals with AD were downloaded from the Alzheimer's Disease Neuroimaging Initiative (ADNI) database. An iteratively reblurred Van Cittert partial volume correction (PVC) method was applied to all preprocessed PET images. MRI images were used for PET spatial normalization and gray matter volume calculation. 18F-AV-1451 PET standardized uptake value ratio (SUVR) was calculated relative to the cerebellum gray matter. The effect of sex and APOE ε4 status on SUVR and gray matter volume were assessed at both region of interest (ROI) and voxelwise levels.
Results: Female APOE ε4 carriers (FACs) had significant higher 18F-AV-1451 SUVRs in the lateral temporal, parietal, posterior cingulate, medial temporal, inferior temporal, entorhinal cortex, amygdala and parahippocampal gyrus regions, and exhibited smaller gray matter volumes in the posterior cingulate, medial temporal, inferior temporal and amygdala regions, as compared to the non-FACs (NFACs) comprised of female APOE ε4 non-carriers, male APOE ε4 carriers and male APOE ε4 non-carriers. Voxelwise analysis revealed forebrain and limbic clusters with greater 18F-AV-1451 SUVRs and lower gray matter volume between FACs compared to the NFACs. Negative correlations between ROI 18F-AV-1451 SUVRs and gray matter volumes were significant after adjusting for age and years of education.
Conclusions: Among elderly individuals with AD, sex modified the effects of the APOE ε4 allele on region-specific tau deposition and gray matter volume. FACs had elevated brain region-specific tau PET SUVR and decreased gray matter volume in comparison to NFACs. The study provides a basis for the use of precision medicine in the diagnosis of AD and evaluation of therapeutics using 18F-AV-1451 PET and structural MRI.
Keywords: Alzheimer's disease, Tau PET, Sex, APOE, Neurodegeneration
Introduction
The apolipoprotein E (APOE) ε4 gene has emerged as a major genetic risk factor for Alzheimer's disease (AD) [1-3]. APOE ε4 is associated with AD-related biological markers including cerebrospinal fluid (CSF) total tau (t-tau), phosphorylated tau (p-tau), brain tau deposition, and gray matter atrophy [4-9].
In addition to APOE ε4, sex also plays an important role in AD risk, with females having a higher lifetime risk of developing AD than males [10, 11]. Sex differences in brain tau pathology and neurodegeneration have been identified in healthy older adults and patients with AD [12-14]. The Lancet Neurology Commission has recently asserted that greater attention to sex differences in AD is essential to advancing both disease prevention and treatment strategies in AD [15].
Numerous CSF and epidemiological evidence support modulatory effects of APOE ε4 on sex-specific disease risk and neuropathology in AD. For example, female APOE ε4 carriers (FACs) have a greater risk of developing AD [16, 17] and far more pronounced disease progression [18]. Studies of CSF t-tau and p-tau have suggested that FACs accumulate more tau pathology than males in clinically normal older adults [19], patients with mild cognitive impairment (MCI) [18], and even mixed diagnostic cohorts (clinically normal older adults, MCI and AD) [13]. In addition, emerging evidence suggests that the cortical tau measured by quantitative positron emission tomography (PET) is temporally and prospectively related to the degree of neurodegeneration and current cognitive status [20-23]. Two recent studies have investigated how sex modulates the effect of APOE ε4 on brain tau deposition using quantitative 18F-AV-1451 PET. One study focused on clinically normal individuals in a 2-cohort study, and showed a significant sex by APOE ε4 interaction effect on regional tau retention [12]. Our previous work reported a significant APOE ε4 by sex interaction effect on regional brain tau deposition in individuals with MCI [24]. These two studies suggest that FACs exhibit brain regions with increased tau deposition among cognitively normal older adults and individuals with MCI.
Cross-sectional structural magnetic resonance imaging (MRI) studies have shown a similar APOE ε4 interaction effect on gray matter atrophy. In MCI, the effect of APOE ε4 on hippocampal volume reduction was stronger in females versus males [25]. Similarly among AD patients, FACs had significantly smaller hippocampal volume compared to female APOE ε4 non-carriers, which, however, was not observed in males [9].
Understanding these APOE ε4 by sex interaction effects may help us identify and apply customized prevention strategies for different populations against AD. The evidence of how sex in association with APOE ε4 affects tau PET and gray matter volume, and whether tau pathology is associated with neurodegeneration in patients with AD remain poorly understood. The objective of the present study was to assess whether sex modulates the effects of the APOE ε4 allele on brain tau deposition and atrophy in older adults with AD.
Methods
Participants
We included 57 participants from the Alzheimer's Disease Neuroimaging Initiative (ADNI) who had (i) a clinical diagnosis of AD, (ii) undergone 18F-AV-1451 tau PET, (iii) high resolution 3D T1-weighted structural MRI, and (iv) APOE ε4 genotyping information. Consent was obtained from all participants prior to the start of the study. For each subject, the most recent tau PET and T1-weighted MRI scan were included. A full list of study inclusion and exclusion criteria can be found at https://adni.loni.usc.edu/wp-content/uploads/2008/07/adni2-procedures-manual.pdf.
PET and MRI data acquisition and processing
Preprocessed 18F-AV-1451 PET images and raw T1-weighted images were downloaded from the ADNI database (http://adni.loni.usc.edu/). The 18F-AV-1451 PET acquisition parameters can be found at http://adni.loni.usc.edu/methods/pet-analysis-method/pet-analysis/. The PET images had been previously aligned, averaged, reoriented and interpolated into a standard image space with uniform voxel size (image volume 160 × 160 × 96, 1.5 × 1.5 × 1.5 mm3 in x, y, z), and then were smoothed to a uniform resolution of 8 mm in full width at half maximum (FWHM) by the ADNI consortium.
As described in our previous studies [24, 26], we further processed the downloaded PET images for partial volume correction (PVC) and spatial normalization using Statistical Parametric Mapping (SPM12, Wellcome Department of Imaging Neuroscience, Institute of Neurology, London, UK) with CAT12 toolbox (CAT12: http://www.neuro.uni-jena.de/cat/) and MATLAB R2019b (The MathWorks Inc). PVC was applied to the processed PET images to correct for or minimize potential underestimation in PET quantification. In brief, an iterative reblurred Van Cittert method [27] was used for PVC on the PET images. The algorithm was implemented using a 3-D Gaussian kernel with 8 mm FWHM, step length α =1.5, and an iteration stop criterion of relative percent change of PVC images < 1%. All PET images with PVC were then coregistered to the corresponding structural MRI images. The MRI images were normalized to standard Montreal Neurologic Institute (MNI) space using SPM12 with a MRI template (image volume: 121×145×121, voxel size: 1.5×1.5×1.5 mm3 in x, y, z). The transformation parameters determined by MRI spatial normalization were then applied to the coregistered PET images for PET spatial normalization. Regions of interest (ROIs) including cerebellum gray matter as a reference tissue were manually drawn on the MRI template using PMOD (version 4.002; PMOD Technologies Ltd., Zürich, Switzerland) in standard MNI space. SUVR images were calculated relative to the cerebellum gray matter. SUVR images without PVC were also generated for reference.
Braak Stage related 12 ROIs, including the entorhinal cortex, parahippocampal, amygdala, inferior temporal, medial temporal, lateral temporal, posterior cingulate, posterior precuneus, parietal, occipital, orbital frontal, and prefrontal, were also defined in the MNI space [22, 24, 26]. The SUVR of each ROI was obtained by calculating the mean of the values within each ROI on the PVC and without PVC PET images in the MNI space. The choice to compute ROI SUVRs in standard space was made to minimize variance related to the variability of ROI volume and shape in native space [24, 26, 28, 29].
Gray matter volume was calculated using the CAT12 toolbox extension of SPM12. CAT12 preprocessing was conducted following the standard default procedure suggested in the manual. First, structural T1-weighted MRI images were segmented into gray matter, white matter and CSF. Then, gray matter segmentations were warped to MNI space with modulated spatial normalization. Finally, the 12 cortical ROIs gray matter volumes were calculated from the modulated gray matter images.
APOE genotyping, CSF t-tau and p-tau assessment
A 10 ml sample of peripheral blood was collected from each subject for direct APOE genotyping. Restriction enzyme isoform genotyping was performed on the extracted DNA to test for the presence of the APOE ε4 genotype, as described previously [30]. APOE ε4 carriers were defined as participants who had one or more ε4 allele (ε4/ ε4, ε4/ ε3 and ε4/ ε2). Those without any ε4 allele were defined as APOE ε4 non-carriers. CSF samples were acquired through lumbar puncture as described in ADNI: http://adni.loni.usc.edu/methods/documents/. The Roche study protocol developed by the UPenn/ADNI Biomarker Laboratory was used for Aβ42, t-tau, and p-tau CSF quantification, as described in previously [31, 32].
Statistical analysis
Recent studies have found no significant differences among non-FACs (NFACs, including female APOE ε4 non-carriers, male APOE ε4 carriers and male APOE ε4 non-carriers) in 18F-AV-1451 ROI SUVRs in cognitively normal adults [12] and individuals with MCI [24]. In the current tau PET study, we first tested the hypothesis that previous results from cognitively normal and MCI cohorts hold true in our AD cohort. Statistical analysis using generalized linear models (GLMs) proc with LS-means/pdiff, implemented in Statistical Analysis System (SAS version 9.4, SAS Institute, Inc.), confirmed that 18F-AV-1451 SUVR did not significantly differ among NFACs in any of the 12 study ROIs. Specifically, no regions were significantly different in an overall test of significance between NFAC groups with P values ranging from 0.19 for the medial temporal to 0.99 for the occipital. Likewise, in pairwise intergroup comparisons (i.e. female APOE ε4 non-carriers vs. male APOE ε4 carriers, female APOE ε4 non-carriers vs. male APOE ε4 non-carriers, and male APOE ε4 carriers vs. male APOE ε4 non-carriers), no regions were significantly different with P values ranging from 0.09 for the inferior temporal female APOE ε4 non-carriers vs. male APOE ε4 non-carriers in comparison to 0.99 for the posterior precuneus female APOE ε4 non-carriers vs. male APOE ε4 carriers comparison. Based on this finding, in order to increase statistical power, all subsequent analyses compared FACs to the NFACs. SAS9.4 and SPM12 were used for all statistical analyses. GLMs adjusted for age and years of education were used to assess group differences in SUVR and gray matter volume for both ROI and voxelwise analyses. For ROI analyses, P < 0.05 was considered significant. For voxelwise analyses, an uncorrected P < 0.001 and cluster size > 100 voxels were defined as significant. Additional statistical results with multiple comparison corrections are discussed in the Discussion section. To investigate whether regional tau retention was associated with neurodegeneration, the correlation between ROIs 18F-AV-1451 SUVRs and gray matter volume was analyzed using partial correlation analysis.
Results
Demographics
A total of 57 AD subjects (mean age: 78.98 ± 9.22 years, 25 females (44%), 34 APOE ε4 carriers (60%)) with 18F-AV-1451 PET and structural MRI scans were included in the study. Demographic characteristics, cognitive function and CSF Aβ42, t-tau and p-tau levels of FACs and NFACs are listed in Table 1. There were no significant differences in age and years of education between males and females for either the APOE ε4 carrier group or the non-carrier group (P > 0.05). Performance on the Mini-Mental State Examination (MMSE), global Clinical Dementia Rating (CDR) and CDR-sum of boxes (CDR-SOB) score (P > 0.05) were not significantly different among the four subgroups, after adjusting for age and years of education. Chi-squared analysis revealed no significant difference in the proportion of individuals with APOE ε4 ε4/ ε4 ε3/ ε4 ε2 genotypes (P = 0.95) between males and females.
Study cohort characteristics
| Parameter | Female APOE ε4+ (n = 15) | Female APOE ε4- (n = 10) | Male APOE ε4+ (n = 19) | Male APOE ε4- (n = 13) |
|---|---|---|---|---|
| Age (years) | 76.58±7.98 | 79.26±11.60 | 79.32±8.63 | 81.06±9.86 |
| Education (years) | 14.73±1.94 | 13.50±2.01 | 17.11±2.62 | 16.00±2.71 |
| MMSE | 21.20±4.59 | 20.10±5.86 | 22.79±4.40 | 22.15±5.16 |
| Global CDR | 1.13±0.48 | 1.20±0.59 | 0.87±0.50 | 0.88±0.42 |
| CDR-SOB | 1.38±1.90 | 3.56±4.27 | 1.46±2.79 | 1.00±1.84 |
| APOE ε4ε4/ε4ε3/ε4ε2 | 5/8/2 | - | 6/11/2 | - |
| CSF Aβ42 (pg/mL) | 589.56±180.47 | 795.35±262.64 | 625.24±195.16 | 722.17±343.62 |
| CSF t-tau (pg/mL) | 433.03±202.33 | 343.43±191.15 | 341.76±109.82 | 333.44±180.90 |
| CSF p-tau (pg/mL) | 43.31±25.37 | 31.60±21.84 | 34.19±11.86 | 33.34±21.58 |
Notes. CDR: Clinical Dementia Rating; CDR-SOB: Clinical Dementia Rating Scale sum of boxes; CSF: cerebrospinal fluid; MMSE: Mini-Mental State Examination.
Sex modifies the APOE ε4 effect on brain tau deposition measured by 18F-AV-1451 PET SUVR
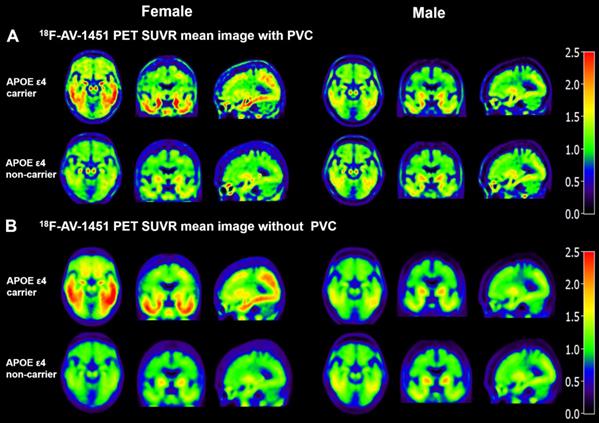
Mean 18F-AV-1451 SUVR images in Figure 1 visually demonstrate that FACs have higher SUVR in both PVC and without PVC images in comparison the NFACs. Compared to the without PVC images (Figure 1B), the mean 18F-AV-1451 SUVR images with PVC in Figure 1A exhibit a higher SUVR contrast between APOE ε4 carriers and non-carriers, consistent with our previous study [22]. Based on this finding, PVC images were retained for subsequent analyses.
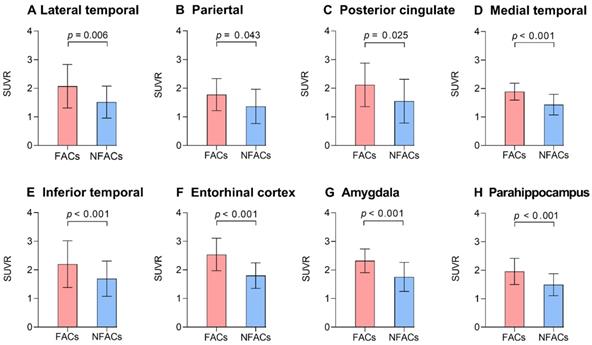
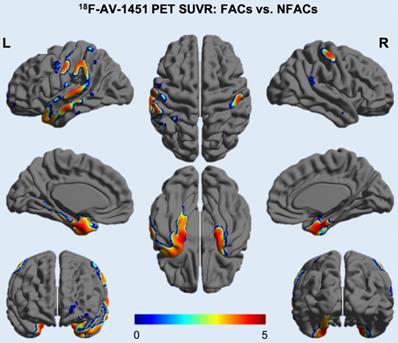
After adjusting for age and years of education, ROI analysis revealed significantly higher ROI SUVRs between FACs compared to the NFACs with AD in the lateral temporal, parietal, posterior cingulate, medial temporal, inferior temporal, entorhinal cortex, amygdala and parahippocampal gyrus (P < 0.05; Figure 2). Voxelwise analysis revealed higher SUVR between FACs compared to the NFACs with AD in clusters corresponding to the bilateral middle temporal, middle temporal pole, inferior temporal, entorhinal cortex, parahippocampal gyrus, amygdala, left superior temporal, parietal, middle occipital, precentral, middle frontal, superior frontal and middle orbitofrontal lobe (P < 0.001, Figure 3, Table 2) after adjusting for age and years of education.
Sex modifies the APOE ε4 effect on gray matter volume measured by structural MRI
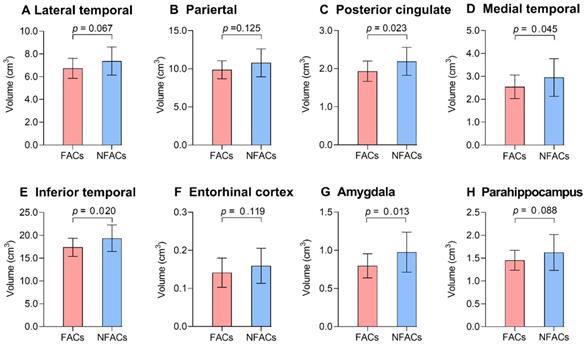
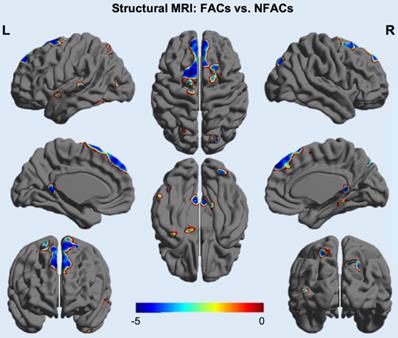
ROI-based analysis revealed significantly lower gray matter volume among FACs compared to the NFACs in the posterior cingulate, medial temporal, inferior temporal and amygdala after adjusting for age and years of education (P < 0.05; Figure 4). In voxelwise analysis, FACs had decreased gray matter volume in clusters located in the left inferior temporal, inferior occipital, middle temporal, middle occipital, amygdala, posterior cingulate; right superior temporal pole, superior occipital, precuneus, bilateral superior-medial frontal, supplementary motor area and superior frontal lobe compared to the NFACs after adjusting for age and years of education (P < 0.001, Figure 5, Table 3).
Mean SUVR images of 18F-AV-1451 PET with and without partial volume correction in individuals with AD. Partial volume corrected SUVR images (A) show increased contrast and spatial resolution compared with the images without PVC (B). Both with and without PVC images visually demonstrate that FACs have higher 18F-AV-1451 SUVR. FACs: female APOE ε4 carriers, PVC: partial volume correction.
The FACs have higher ROI 18F-AV-1451 PET SUVRs relative to NFACs with AD. Mean (±standard deviation) of ROI 18F-AV-1451 PET SUVR for FACs (red) and NFACs with AD (blue) are depicted. P value was determined by a generalized linear model with age, years of education included as covariates. FACs: female APOE ε4 carriers, NFACs: non-FACs, SUVR: standardized uptake value ratio.
18F-AV-1451 SUVR voxelwise difference between FACs and NFACs with AD. T values are expressed in blue-red colors from 0 to 5 (P < 0.001, uncorrected, adjusted for age and years of education). FACs: female APOE ε4 carriers, NFACs: non-FACs.
Association between tau SUVR and gray matter volume
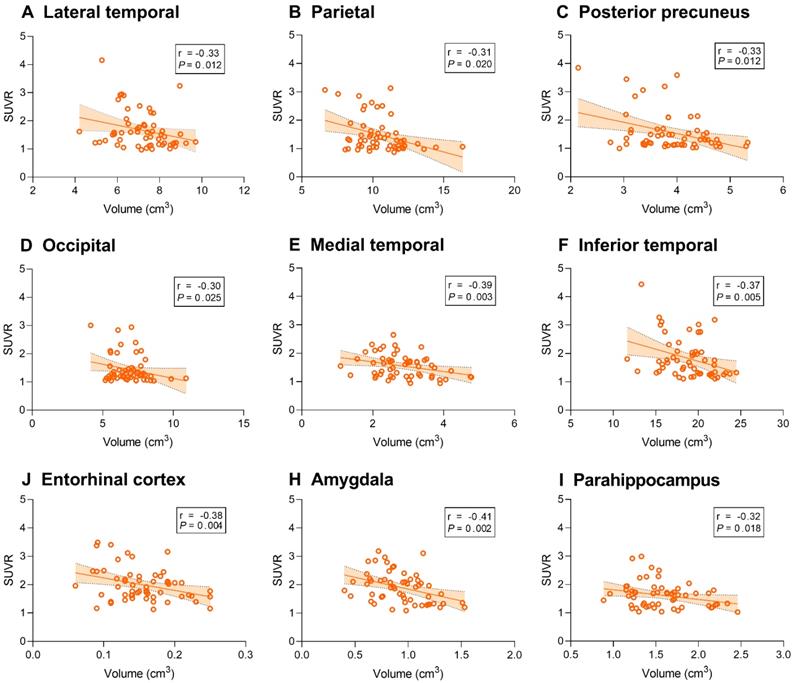
We then sought to identify associations between tau deposition and gray matter volume. 18F-AV-1451 SUVR was associated with gray matter volume of the temporal, parietal, posterior precuneus, occipital, entorhinal cortex, amygdala and parahippocampal regions (P < 0.05, Figure 6).
Discussion
By extending previous work on APOE ε4-sex interaction effects on brain tau deposition in cognitively normal and MCI cohorts [12, 24], our study revealed that sex also modifies the effect of APOE ε4 on brain tau deposition and atrophy in individuals with AD using data from the ADNI consortium. Recent work on clinically normal individuals from the Harvard Aging Brain Study found no sex-APOE ε4 interaction effect on regional tau deposition. However, within the ADNI cohort, a sex by APOE ε4 interaction effect on brain tau deposition was previously found in the early Braak stages (Ⅰ-Ⅲ) regions including the entorhinal cortex, inferior temporal, amygdala, fusiform and parahippocampal gyrus [12, 33]. For individuals with MCI, sex-stratified analysis showed that FACs had higher tau SUVR in the entorhinal cortex, amygdala, fusiform, parahippocampal gyrus, posterior cingulate, occipital, temporal, parietal and posterior precuneus (Braak stages Ⅰ-Ⅴ) compared to NFACs [24]. In this study, we focused on older adults with AD, and found that FACs had more tau deposition in the temporal, parietal, post cingulate, entorhinal cortex, amygdala, parahippocampal gyrus (Braak stages Ⅰ-Ⅴ) regions compared to the NFACs. Overall, our results add to a growing body of work demonstrating an APOE ε4 and sex interaction effect in AD whereby FACs exhibit greater brain region-specific tau deposition compared to NFACs across sub-clinical and clinical stages of AD.
Recent metabolic and transcriptomic data add potential mechanistic explanations to the findings of this study [34-37]. For example, prior work has found that specific metabolic effects were limited to FACs. A recent study found that, among FACs, higher acylcarnitine C10 was associated with higher CSF p-tau, and higher proline levels were associated with reduced brain glucose uptake. These effects were not observed in the NFACs, suggesting that FACs experience greater impairment of mitochondrial energy production compared to NFACs with AD [36]. This provides an additional line of support for the findings drawn in this work. Moreover, in-vitro and transcriptomic data has suggested that sex modulates neuroinflammation, another risk factor for AD, with inflammatory dysregulation being stronger in females with AD [37, 38]. APOE ε4 also triggers neuroinflammatory cascades that cause neurovascular dysfunction resulting in leakage of blood derived toxic proteins into the brain [39]. These data suggest a potential model of AD pathogenesis in which the female sex and APOE ε4 allele contribute to neuroinflammatory damage to the brain and brain microvasculature, leading to increased tau and brain atrophy in FACs.
Strikingly, although FACs had greater tau deposition, we observed no differences in MMSE score, global CDR and CDR-SOB between FACs and NFACs. The implication that FACs exhibit increased tau deposition without commensurate cognitive decline warrants further investigation. This may suggest that FACs experience greater cognitive resilience to the effects of tauopathy compared to males [24]. This is supported by a 18F-FDG PET study in which cognitively normal female adults had a younger metabolic brain age compared with males [40].
We also evaluated sex by APOE ε4 interaction effects on brain region-specific atrophy. FACs had a significant lower gray matter volume in the cingulate, medial temporal, inferior temporal and amygdala cortex than the NFACs with AD after adjusting for age and years of education. Previous studies have demonstrated that APOE ε4 allele is associated with significantly lower hippocampal gray matter volume in females with MCI and AD, but not in males [9, 25]. Consistent with past findings, our ROI-based and voxelwise analysis showed that FACs had reduced gray matter volume in several cortical regions compared with the NFACs. Moreover, we also found negative correlations between brain regional tau PET uptake and grey matter volume, which is consistent with existing studies [41, 42]. This suggests that tau pathology is a major driver of local and distant cortical atrophy [20, 43].
Clusters with increased tau deposition among FACs with AD
| Clusters | Cluster size | Atlas Coordinates | Z score | P value | |||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| Middle temporal/ Middle temporal pole/Parahippocampal/Entorhinal cortex/Amygdala/Inferior parietal/Superior parietal/Superior temporal/Middle occipital/Precentral. | L | 9892 | -42 | -54 | 60 | 6.27 | < 0.001 |
| Parahippocampal/Middle temporal pole/Inferior temporal/Middle temporal/Entorhinal cortex/Amygdala. | R | 1020 | 16.5 | -4.5 | -21 | 5.87 | < 0.001 |
| Middle frontal/ Superior frontal/Middle orbitofrontal. | L | 1416 | -43 | 46 | 21 | 5.00 | < 0.001 |
Notes: The data were extracted from voxels associated with maximally significant 18F-AV451 PET SUVR increases in FACs compared with the NFACs with AD, after controlling for age and years of education. Cluster locations correspond to the brain maps shown in Figure 3 and correspond to P < 0.001. Atlas coordinates were obtained from the AAL atlas. The X, Y and Z coordinates are shown in the MNI space. FACs: female APOE ε4 carriers.
The FACs had lower ROI gray matter volume relative to NFACs with AD. Mean (± standard deviation) of ROI gray matter volume for FACs (red) and NFACs (blue) with AD are depicted. P values were determined by a generalized linear model with age, years of education were included as covariates. For consistency, the regions displayed are those in which FACs have significantly higher ROI 18F-AV-1451 PET SUVRs from Figure 2. FACs: female APOE ε4 carriers, NFACs: non-FACs, SUVR: standardized uptake value ratio.
Voxelwise difference of gray matter density between FACs and NFACs with AD. T values are expressed along the blue-red scale from -5 to 0 (P < 0.001, adjusted for age and years of education). FACs: female APOE ε4 carriers, NFACs: non-FACs.
Clusters with decreased gray matter volume among FACs with AD
| Clusters | Cluster size | Atlas coordinates (peak) | Z score | P value | |||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| Inferior temporal/ Occipital. | L | 653 | -54 | -52.5 | -22.5 | -4.04 | < 0.001 |
| Superior temporal pole | R | 135 | 58.5 | 15 | -13.5 | -3.78 | < 0.001 |
| Superior temporal | L | 204 | -63 | -3 | -9 | -3.94 | < 0.001 |
| Middle temporal | L | 446 | -69 | -30 | 1.5 | -4.10 | < 0.001 |
| Amygdala | L | 104 | -17.5 | -4.5 | -16.5 | -4.35 | < 0.001 |
| Middle occipital | L | 137 | -45 | -82.5 | 10.5 | -4.16 | < 0.001 |
| Superior occipital/ Precuneus. | R | 762 | 27 | -84 | 39 | -4.73 | < 0.001 |
| Posterior cingulate | L | 471 | -3 | -46.5 | 19 | -5.71 | < 0.001 |
| Superior-medial frontal/Supplementary motor area/Superior frontal. | L/R | 3003 | 1.5 | 54 | 42 | -5.41 | < 0.001 |
Notes: The data were extracted from voxels associated with maximally significant gray matter volume decreases in FACs compared to the NFACs with AD, after controlling for age and years of education. Listed locations correspond to the brain maps shown in Figure 5 and correspond to P < 0.001. Atlas coordinates were obtained from the AAL atlas. The X, Y and Z coordinates are shown in the MNI space. FACs: female APOE ε4 carriers, NFACs: non-FACs.
In the current study, we pooled data from female APOE ε4 non-carriers, male APOE ε4 carriers and male APOE ε4 non-carriers into a group labeled NFACs. To evaluate whether similar results persist when using not pooled data, we also analyzed group comparisons for 18F-AV-1451 SUVRs between FACs and female APOE ε4 non-carriers, male APOE ε4 carriers, male APOE ε4 non-carriers respectively. The results from both “not pooled” and “pooled” data were highly consistent across 6 out of the 8 originally significant ROIs including the lateral temporal, medial temporal, inferior temporal, entorhinal cortex, amygdala and parahippocampal gyrus. In the “non-pooled” data, the SUVRs of posterior cingulate and parietal showed a marginal P value between FACs and female APOE ε4 non-carriers, male APOE ε4 carriers, male APOE ε4 non-carriers (posterior cingulate: P = 0.07, 0.10, 0.08 and parietal: P = 0.12, 0.13, 0.09, respectively), but significant group differences were observed after “pooling” the data (P = 0.025 and 0.043). The findings suggest that the increased sample size from pooling data enhanced the study's statistical power to discover FAC-associated effects.
Limitations of this study must be considered when interpreting the results. First, our analysis was an observation study within the multicenter ADNI cohort. The high biological and physiological heterogeneity in AD related to age, sex and disease stage, and the relatively small number of AD subjects (15 FACs and 42 NFACs, all of whom received 18F-AV-1451 tau PET scans and APOE ε4 genotyping) may limit the generalizability of results from the study. For example, in our cohort the age range was 55-93 (78.98 ± 9.22 years) and CDR range was 0.5-2.0. Given that the effects of sex and APOE ε4 are known to vary significantly across disease stage and age groups [44, 45], our results should be interpreted within the age range in this cohort. It should be noted, however, that the frequency of FACs was 26.3%, which is comparable to an existing study [46]. Further, due to its robustness within smaller sample sizes, a GLM was used to assess group differences in tau deposition and brain atrophy at both ROI and voxelwise levels. We also evaluated the probability that the current results are affected by sample size by calculating the statistical power for tau SUVR to distinguish FACs and NFACs. The statistical power ranged from 0.725 to 0.999 with power > 0.8 in 10 out of the 12 study ROIs. The statistical power for gray matter volume to distinguish between the two groups ranged from 0.220 to 0.999, with power > 0.8 in 5 out of the 12 study ROIs. This suggests that tau-PET may be more sensitive than spatial MRI to elucidate APOE ε4 and sex effects in AD.
Considering the exploratory nature of our study, our sample size and the correlated nature of the tau positive study ROIs [47, 48], we choose to minimize the probability of false negatives and include results without multiple comparisons correction in the main results. We recognize that this will increase our false positive rate and have evaluated robustness to false positives by applying the Benjamini-Hochberg method for ROI based analyses and family wise error (FWE) correction for voxelwise analyses. For the ROI-based 18F-AV-1451 analysis, all regions remained significant at false discovery rates (FDR) < 0.1 [24]. For the ROI-based gray matter analysis, three regions i.e. the amygdala, inferior temporal and posterior cingulate remained significant at FDR < 0.1. All regions remained significant at FDR < 0.2. For the voxelwise 18F-AV-1451 analysis, clusters in the right inferior temporal, left amygdala, inferior parietal, superior parietal, bilateral entorhinal cortex and parahippocampal gyrus regions remained significant after FWE correction. For the voxelwise gray matter analyses, no clusters survived FWE correction.
Correlation between regional 18F-AV-1451 SUVR and gray matter volume in AD participants. Line graphs showing correlation between regional gray matter volume (cm3) and 18F-AV-1451 SUVR. Fitted lines, P-values, and 95% confidence intervals are displayed from partial correlation analysis. SUVR: standardized uptake value ratio.
Further, we acknowledge that the current work is based on the ADNI cohort and may not generalize to other AD patient populations. Future analyses should be conducted in multi institutional cohorts beyond ADNI with larger sample sizes to validate this preliminary work.
Conclusion
In summary, sex modifies the effects of the APOE ε4 allele on brain region-specific tau deposition and gray matter volume in older adults with AD. Specifically, FACs exhibited elevated brain region-specific tau PET SUVR and deceased gray matter volume. Results from this study advance our understanding of the effect of sex and APOE ε4 in promoting tau pathology in AD. Importantly, this work highlights the importance of considering sex and APOE ε4 in biomarker development, clinical trial endpoint evaluation, and mechanistic studies in AD using quantitative 18F-AV-1451 PET.
Abbreviations
AD: Alzheimer's disease; ADNI: Alzheimer's disease neuroimaging initiative; APOE ε4: apolipoprotein E type 4 allele; CDR: Clinical Dementia Rating; CDR-SOB: Clinical Dementia Rating Scale sum of boxes; CSF: cerebrospinal fluid; FWHM: full width at half maximum; GLM: generalized linear model; MCI: mild cognitive impairment; MMSE: Mini-Mental State Examination; MNI: Montreal Neurological Institute; MRI: magnetic resonance imaging; PET: positron emission tomography; p-tau: phosphorylated tau; PVC: partial volume correction; ROI: region of interest; SAS: Statistical Analysis System; SPM: Statistical Parametric Mapping; SUVR: standardized uptake value ratio; t-tau: total tau.
Acknowledgements
This study was partially supported by Mallinckrodt Institute of Radiology, Washington University in St. Louis School of Medicine. The authors acknowledge Drs. Manu Goyal and Andrei Vlassenko for their thoughtful discussions on sex differences in brain resilience.
Group Information: Data used in preparation of this article were obtained from the Alzheimer's Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at: https://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf.
Author contributions
J.L, and Y.Z had full access to do all the data in the study and take responsibility for the integrity of the data and the accuracy of data analysis. Study concept and design: S.Y, J.L, and Y.Z. Acquisition, analysis, or interpretation of data: All authors. Drafting and critical revision of the manuscript for important intellectual content: All authors. Administrative, technical, or material support: J.L and Y.Z.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Genin E, Hannequin D, Wallon D, Sleegers K, Hiltunen M, Combarros O. et al. APOE and Alzheimer disease: a major gene with semi-dominant inheritance. Mol Psychiatry. 2011;16(9):903-7
2. Gaugler J, James B, Johnson T, Marin A, Weuve J. 2019 Alzheimer's disease facts and figures. Alzheimers Dement. 2019;15(3):321-87
3. Michaelson DM. APOE ε4: The most prevalent yet understudied risk factor for Alzheimer's disease. Alzheimers Dement. 2014;10(6):861-8
4. Darreh-Shori T, Siawesh M, Mousavi M, Andreasen N, Nordberg A. Apolipoprotein ε4 modulates phenotype of butyrylcholinesterase in CSF of patients with Alzheimer's disease. J Alzheimers Dis. 2012;28(2):443-58
5. Agostaa F, Vossela KA, Millera BL, Migliaccioa R, Bonaseraa SJ, Filippib M. et al. Apolipoprotein E 4 is associated with disease-specific effects on brain atrophy in Alzheimer's disease and frontotemporal dementia. Proc Natl Acad Sci U S A. 2009;106(6):2018-22
6. Shi Y, Yamada K, Liddelow SA, Smith ST, Zhao L, Luo W. et al. ApoE4 markedly exacerbates tau-mediated neurodegeneration in a mouse model of tauopathy. Nature. 2017;549(7673):523-7
7. Tiraboschi P, Hansen LA, Masliah E, Alford M, Thal LJ, Corey-Bloom J. Impact of APOE genotype on neuropathologic and neurochemical markers of Alzheimer disease. Neurology. 2004;62(11):1977-83
8. Haller S, Montandon M-L, Rodriguez C, Ackermann M, Herrmann F, Giannakopoulos P. APOE* E4 is associated with gray matter loss in the posterior cingulate cortex in healthy elderly controls subsequently developing subtle cognitive decline. AJNR Am J Neuroradiol. 2017;38(7):1335-42
9. Liu Y, Paajanen T, Westman E, Wahlund L-O, Simmons A, Tunnard C. et al. Effect of APOE ε4 allele on cortical thicknesses and volumes: the AddNeuroMed study. J Alzheimers Dis. 2010;21(3):947-66
10. Barnes LL, Wilson RS, Bienias JL, Schneider JA, Evans DA, Bennett DA. Sex differences in the clinical manifestations of Alzheimer disease pathology. Arch Gen Psychiatry. 2005;62(6):685-91
11. Nebel RA, Aggarwal NT, Barnes LL, Gallagher A, Goldstein JM, Kantarci K. et al. Understanding the impact of sex and gender in Alzheimer's disease: A call to action. Alzheimers Dement. 2018;14(9):1171-83
12. Buckley RF, Mormino EC, Rabin JS, Hohman TJ, Landau S, Hanseeuw BJ. et al. Sex differences in the association of global amyloid and regional tau deposition measured by positron emission tomography in clinically normal older adults. JAMA Neurol. 2019;76(5):542-51
13. Hohman TJ, Dumitrescu L, Barnes LL, Thambisetty M, Beecham G, Kunkle B. et al. Sex-specific association of Apolipoprotein E with cerebrospinal fluid levels of tau. JAMA Neurol. 2018;75(8):989-98
14. Malpetti M, Ballarini T, Presotto L, Garibotto V, Tettamanti M, Perani D. Gender differences in healthy aging and Alzheimer's Dementia: A 18F-FDG-PET study of brain and cognitive reserve. Hum Brain Mapp. 2017;38(8):4212-27
15. Mazure CM, Swendsen J. Sex differences in Alzheimer's disease and other dementias. Lancet Neurol. 2016;15(5):451-2
16. Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW. et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261(5123):921-3
17. Saunders AM, Strittmatter WJ, Schmechel D, George-Hyslop PH, Pericak-Vance MA, Joo SH. et al. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer's disease. Neurology. 1993;43(8):1467-72
18. Altmann A, Tian L, Henderson VW, Greicius MD. Sex modifies the APOE-related risk of developing Alzheimer disease. Ann Neurol. 2014;75(4):563-73
19. Damoiseaux JS, Seeley WW, Zhou J, Shirer WR, Coppola G, Karydas A. et al. Gender modulates the APOE epsilon4 effect in healthy older adults: convergent evidence from functional brain connectivity and spinal fluid tau levels. J Neurosci. 2012;32(24):8254-62
20. La Joie R, Visani AV, Baker SL, Brown JA, Bourakova V, Cha J. et al. Prospective longitudinal atrophy in Alzheimer's disease correlates with the intensity and topography of baseline tau-PET. Sci Transl Med. 2020 12(524)
21. Brier MR, Gordon B, Friedrichsen K, McCarthy J, Stern A, Christensen J. et al. Tau and Abeta imaging, CSF measures, and cognition in Alzheimer's disease. Sci Transl Med. 2016;8(338):338ra66
22. Zhao Q, Liu M, Ha L, Zhou Y. Quantitative 18F-AV1451 brain tau PET imaging in cognitively normal older adults, mild cognitive impairment, and Alzheimer's disease patients. Front Neurol. 2019 10486
23. Maass A, Lockhart SN, Harrison TM, Bell RK, Mellinger T, Swinnerton K. et al. Entorhinal tau pathology, episodic memory decline, and neurodegeneration in aging. J Neurosci. 2018;38(3):530-43
24. Liu M, Paranjpe MD, Zhou X, Duy PQ, Goyal MS, Benzinger TLS. et al. Sex modulates the ApoE epsilon4 effect on brain tau deposition measured by 18F-AV-1451 PET in individuals with mild cognitive impairment. Theranostics. 2019;9(17):4959-70
25. Fleisher A, Grundman M, Jack CR, Petersen RC, Taylor C, Kim HT. et al. Sex, apolipoprotein E ε4 status, and hippocampal volume in mild cognitive impairment. Arch Neurol. 2005;62(6):953-7
26. Paranjpe MD, Chen X, Liu M, Paranjpe I, Leal JP, Wang R. et al. The effect of ApoE epsilon4 on longitudinal brain region-specific glucose metabolism in patients with mild cognitive impairment: a FDG-PET study. Neuroimage Clin. 2019 22101795
27. Tohka J, Reilhac A. Deconvolution-based partial volume correction in Raclopride-PET and Monte Carlo comparison to MR-based method. Neuroimage. 2008;39(4):1570-84
28. Tudorascu DL, Minhas DS, Lao PJ, Betthauser TJ, Yu Z, Laymon CM. et al. The use of Centiloids for applying [11C] PiB classification cutoffs across region-of-interest delineation methods. Alzheimers Dement. 2018:10332-9
29. Gottesman RF, Schneider AL, Zhou Y, Coresh J, Green E, Gupta N. et al. Association between midlife vascular risk factors and estimated brain amyloid deposition. JAMA. 2017;317(14):1443-50
30. Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res. 1990;31(3):545-8
31. Bittner T, Zetterberg H, Teunissen CE, Ostlund RE Jr, Militello M, Andreasson U. et al. Technical performance of a novel, fully automated electrochemiluminescence immunoassay for the quantitation of beta-amyloid (1-42) in human cerebrospinal fluid. Alzheimers Dement. 2016;12(5):517-26
32. Hansson O, Seibyl J, Stomrud E, Zetterberg H, Trojanowski JQ, Bittner T. et al. CSF biomarkers of Alzheimer's disease concord with amyloid-beta PET and predict clinical progression: A study of fully automated immunoassays in BioFINDER and ADNI cohorts. Alzheimers Dement. 2018;14(11):1470-81
33. Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239-59
34. Hobel Z, Isenberg AL, Raghupathy D, Mack W, Pa J. APOE ɛ4 gene dose and sex effects on Alzheimer's disease MRI biomarkers in older adults with mild cognitive impairment. J Alzheimers Dis. 2019;71(2):647-58
35. Liu CC, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol. 2013;9(2):106-18
36. Arnold M, Nho K, Kueider-Paisley A, Massaro T, Huynh K, Brauner B. et al. Sex and APOE ε4 genotype modify the Alzheimer's disease serum metabolome. Nat Commun. 2020;11(1):1-12
37. Paranjpe MD, Belonwu S, Wang JK, Oskotsky T, Gupta A, Taubes A. et al. Sex-specific cross tissue Meta-analysis identifies immune dysregulation in women with Alzheimer's disease. bioRxiv. 2020
38. Hanamsagar R, Bilbo SD. Sex differences in neurodevelopmental and neurodegenerative disorders: focus on microglial function and neuroinflammation during development. J Steroid Biochem Mol Biol. 2016:160127-33
39. Bell RD, Winkler EA, Singh I, Sagare AP, Deane R, Wu Z. et al. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature. 2012;485(7399):512-6
40. Goyal MS, Blazey TM, Su Y, Couture LE, Durbin TJ, Bateman RJ. et al. Persistent metabolic youth in the aging female brain. Proc Natl Acad Sci U S A. 2019;116(8):3251-5
41. Iaccarino L, Tammewar G, Ayakta N, Baker SL, Bejanin A, Boxer AL. et al. Local and distant relationships between amyloid, tau and neurodegeneration in Alzheimer's Disease. Neuroimage Clin. 2018:17452-64
42. Sone D, Imabayashi E, Maikusa N, Okamura N, Furumoto S, Kudo Y. et al. Regional tau deposition and subregion atrophy of medial temporal structures in early Alzheimer's disease: A combined positron emission tomography/magnetic resonance imaging study. Alzheimers Dement. 2017:935-40
43. Das SR, Xie L, Wisse LE, Ittyerah R, Tustison NJ, Dickerson BC. et al. Longitudinal and cross-sectional structural magnetic resonance imaging correlates of AV-1451 uptake. Neurobiol Aging. 2018:6649-58
44. Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R. et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease: a meta-analysis. JAMA. 1997;278(16):1349-56
45. Vermunt L, Sikkes SA, van den Hout A, Handels R, Bos I, van der Flier WM. et al. Duration of preclinical, prodromal, and dementia stages of Alzheimer's disease in relation to age, sex, and APOE genotype. Alzheimers Dement. 2019;15(7):888-98
46. Neu SC, Pa J, Kukull W, Beekly D, Kuzma A, Gangadharan P. et al. Apolipoprotein E genotype and sex risk factors for Alzheimer disease: a meta-analysis. JAMA Neurol. 2017;74(10):1178-89
47. Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239-59
48. Franzmeier N, Rubinski A, Neitzel J, Kim Y, Damm A, Na DL. et al. Functional connectivity associated with tau levels in ageing, Alzheimer's, and small vessel disease. Brain. 2019;142(4):1093-107
Author contact
![]() Corresponding author: Yun Zhou, Mallinckrodt Institute of Radiology, Washington University in St. Louis School of Medicine, 510 Kingshighway Blvd., St.Louis, MO 63110, USA. Tel: (314)2737792; Fax: (314)3628555; E-mail: yunzhouedu, ORCID: https://orcid.org/0000-0001-9135-336X; Jie Lu, Department of Radiology, Xuanwu Hospital, Capital Medical University, Beijing, China; 45 Changchunjie, Xicheng District, Beijing 100053, China. Tel: 0186-1083198379; Fax: 0186-1083198376; E-mail: imaginglucom, ORCID: https://orcid.org/0000-0003-0425-3921.
Corresponding author: Yun Zhou, Mallinckrodt Institute of Radiology, Washington University in St. Louis School of Medicine, 510 Kingshighway Blvd., St.Louis, MO 63110, USA. Tel: (314)2737792; Fax: (314)3628555; E-mail: yunzhouedu, ORCID: https://orcid.org/0000-0001-9135-336X; Jie Lu, Department of Radiology, Xuanwu Hospital, Capital Medical University, Beijing, China; 45 Changchunjie, Xicheng District, Beijing 100053, China. Tel: 0186-1083198379; Fax: 0186-1083198376; E-mail: imaginglucom, ORCID: https://orcid.org/0000-0003-0425-3921.
 Global reach, higher impact
Global reach, higher impact