13.3
Impact Factor
Theranostics 2020; 10(22):10326-10340. doi:10.7150/thno.45924 This issue Cite
Research Paper
PML-RARα interaction with TRIB3 impedes PPARγ/RXR function and triggers dyslipidemia in acute promyelocytic leukemia
1. National Clinical Research Center for Metabolic Disease, Department of Metabolism and Endocrinology, the Second Xiangya Hospital, Central South University, Changsha, Hunan, 410011, China.
2. Department of Hematology & Institute of Hematology, Zhejiang Province Key Laboratory of Hematology Oncology Diagnosis and Treatment, The First Affiliated Hospital, Zhejiang University, Hangzhou, Zhejiang, 310058, China.
3. Immunology and Cancer Pharmacology Group, State Key Laboratory of Bioactive Substance and Function of Natural Medicines, Institute of Materia Medica, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, 100050, China.
4. NHC Key Laboratory of Biotechnology of Antibiotics, Institute of Medicinal Biotechnology, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing, 100050, China.
5. Department of Hematology, Affiliated Hospital of Xuzhou Medical University, Xuzhou, Jiangsu, 230031 China.
#These authors contributed equally to this work.
Received 2020-3-12; Accepted 2020-7-16; Published 2020-8-15
Abstract
Although dyslipidemia commonly occurs in patients with acute promyelocytic leukemia (APL) in response to anti-APL therapy, the underlying mechanism and the lipid statuses of patients with newly diagnosed APL remain to be addressed.
Methods: We conducted a retrospective study to investigate the lipid profiles of APL patients. PML-RARα transgenic mice and APL cells-transplanted mice were used to assess the effects of APL cells on the blood/liver lipid levels. Subsequently, gene set enrichment analysis, western blot and dual luciferase reporter assay were performed to examine the role and mechanism of PML-RARα and TRIB3 in lipid metabolism regulation in APL patients at pretreatment and after induction therapy.
Results: APL patients exhibited a higher prevalence of dyslipidemia before anti-APL therapy based on a retrospective study. Furthermore, APL cells caused secretion of triglycerides, cholesterol, and PCSK9 from hepatocytes and degradation of low-density lipoprotein receptors in hepatocytes, which elevated the lipid levels in APL cell-transplanted mice and Pml-Rarα transgenic mice. Mechanistically, pseudokinase TRIB3 interacted with PML-RARα to inhibit PPARγ activity by interfering with the interaction of PPARγ and RXR and promoting PPARγ degradation. Thus, reduced PPARγ activity in APL cells decreased leptin but increased resistin expression, causing lipid metabolism disorder in hepatocytes and subsequent dyslipidemia in mice. Although arsenic/ATRA therapy degraded PML-RARα and restored PPARγ expression, it exacerbated dyslipidemia in APL patients. The elevated TRIB3 expression in response to arsenic/ATRA therapy suppressed PPARγ activity by disrupting the PPARγ/RXR dimer, which resulted in dyslipidemia in APL patients undergoing therapy. Indeed, the PPAR activator not only enhanced the anti-APL effects of arsenic/ATRA by suppressing TRIB3 expression but also reduced therapy-induced dyslipidemia in APL patients.
Conclusion: Our work reveals the critical role of the PML-RARα/PPARγ/TRIB3 axis in the development of dyslipidemia in APL patients, potentially conferring a rationale for combining ATRA/arsenic with the PPAR activator for APL treatment.
Keywords: AML, Cancer, leukemia, lipid metabolism, tribbles
Introduction
Acute promyelocytic leukemia (APL) is the M3 subtype of acute myelogenous leukemia (AML), which is driven by a chimeric PML-RAPα oncoprotein [1]. Although increased body mass index (BMI) and a high prevalence of obesity were reported in patients with APL [2-6], the status of the serum lipid profile in newly diagnosed APL patients remains unclear. All-trans retinoic acid (ATRA) and arsenic trioxide (As2O3) have long been used successfully against APL [7-9]. However, in recent years, more attention has been paid to ATRA-induced hypertriglyceridemia in APL patients undergoing ATRA therapy [10-13]. Two mechanisms are assumed to account for the hypertriglyceridemia induced by ATRA. First, ATRA stimulation increases the synthesis of cholesterol and triglycerides in the liver to elevate the blood lipid levels of APL patients [10]. Second, metabolites, including cytokines and adipokines, produced by APL cells may contribute to ATRA-induced hypertriglyceridemia [14, 15]. However, the molecular mechanism of anti-APL therapy-mediated dyslipidemia remains elusive and needs to be further clarified.
As a nuclear receptor with transcription factor functions, peroxisome proliferator-activated receptor-γ (PPARγ) controls lipid and glucose metabolism by forming PPARγ-retinoid X receptor (RXR) heterodimers to bind a PPAR-response element (PPRE) [16]. Retinoic acid receptors (RARs) are ligand-controlled transcription factors that act as heterodimers with RXRs to regulate cell growth and survival and are also implicated in the pathogenesis of metabolic diseases [17]. In APL, the oncoprotein PML-RARα can heterodimerize with RXRs, which bind strongly to retinoic acid response elements and represses the transcription of RAR targets [18, 19]. Given that crosstalk exists between the nuclear receptors PPARγ, RARs and RXRs and that PML-RARα and PPARγ share the same partner, RXRs, we presumed that PML-RARα contributes to abnormal lipid metabolism by competing with PPARγ for RXR partners to inhibit PPARγ target genes.
Tribbles homologue 3 (TRIB3), a member of the pseudokinase family, acts as a stress sensor that responds to a diverse range of stressors, including inflammatory, metabolic and endoplasmic reticulum (ER) stress [20-22]. Our recent study reported that TRIB3 promotes APL progression by interacting with the oncoprotein PML-RARα and inhibiting p53-mediated senescence [23, 24]. Increases in TRIB3 expression induced by ATRA or arsenic treatment decreased the therapeutic efficacy of treatment [23]. TRIB3 functions as a metabolic stress factor to participate in the regulation of lipid and glucose metabolism by interacting with the E3 ubiquitin ligase COP1, decreasing phospho-AKT and negatively regulating PPARγ transcriptional activity [25-27]. Therefore, we herein hypothesize that the increased TRIB3 expression functions together with PML-RARα to participate in the regulation of dyslipidemia in patients with APL. We first conducted a retrospective study to investigate the lipid profiles of an adequate sample of APL patients. Furthermore, we examined the roles and mechanisms of PML-RARα and TRIB3 in lipid metabolism in APL patients before treatment and after induction therapy. Overall, our study not only defines a mechanism by which the crosstalk of PML-RARα/PPARγ/TRIB3 contributes to the abnormal lipid metabolism associated with APL but also provides a rationale for the combination of ATRA/arsenic with PPAR activator for APL therapy.
Materials and Methods
Patients and samples
We conducted a retrospective study of 120 patients with AML (APL and non-APL) at our center from January 2014 through June 2016. The eligibility criteria included patients with an age ranging from 15 to 65 years old and newly diagnosed AML. APL patients received ATRA and arsenic, and non-APL patients received idarubicin (10 mg/m2/d × 3 days) or daunorubicin (45 mg/m2/d × 3 days) and cytarabine (100 mg/m2/d × 7 days) as induction therapy. In addition to age, gender, height, weight and hematological parameters, the total cholesterol (TC), triglyceride (TG), high-density lipoprotein (HDL) cholesterol, and low-density lipoprotein (LDL) cholesterol concentrations were measured before and after induction therapy in all patients.
Intravenous blood was collected from all subjects after 10 ± 2 h of fasting to measure serum lipids. Blood samples were collected in vials containing an EDTA anticoagulant agent. The plasma was promptly separated (< 4 h after collection of whole blood). We used an Abbott ARCHITECT c 16000 instrument and TG, TC, HDL, and LDL test kits (Merit Choice Bioengineering (Beijing) Co., Ltd.), which used the GPO-PAP, CHOP-PAP, catalase clearance and surfactant clearance methods, respectively. Abnormal lipid status was determined by utilizing criteria established by the expert panel of the National Cholesterol Education Program (NCEP), Adult Treatment Panel III (ATP III). The cut-off values, including the upper limits of normal, for TGs, TC, and LDL were 1.7 mmol/L (150 mg/dL), 5.2 mmol/L (200 mg/dL), and 3.4 mmol/L (130 mg/dL), respectively, and the lower limit of normal for HDL was 1.04 mmol/L (60 mg/dL). Informed consent was obtained from all participants in accordance with the Declaration of Helsinki. The procedure was approved by the Ethics Committee of the Institute of Hematology and Blood Diseases Hospital of PUMC (KT2019055-EC-1) and the institutional review board at Affiliated Hospital of Xuzhou Medical University. Patient-related information is shown in Supplementary Table 1.
Definitions of variables
According to the European Society of Cardiology (ESC)/European Atherosclerosis Society (EAS) Guidelines for the Management of Dyslipidemias, hypertriglyceridemia is defined as TGs >1.7 mmol/L (150 mg/dL). For TG-based analysis, the study groups were categorized into two major TG groups: TG group-1 (TG ≤ 1.7 mmol/L) and TG group-2 (TG > 1.7 mmol/L). BMI was calculated as weight in kilograms/(height in meters)2, and the current WHO criteria were used to categorize patients as underweight/normal (BMI < 25 kg/m2) or overweight/obese (BMI ≥ 25 kg/m2). The initial white blood cell (WBC) count was evaluated and adjusted for the APL patients as follows: WBC counts ≤ 10 × 109/L and > 10 × 109/L for the low- and high-risk categories, respectively [28].
Animal Studies
The myeloid cell-specific Pml-Rarα knockin (Pml-RarαKI), Pml-RarαKI Trib3 knockin (PR-T3KI), and Pml-RarαKI Trib3 knockout (PR-T3KO) transgenic mice (C57BL/6, male) were constructed as described previously [23]. hMRP8-Pml-Rarα transgenic mice were obtained from Kan-kan Wang's laboratory [29, 30]. These mice were maintained in the animal facility at the Institute of Materia Medica under specific-pathogen-free (SPF) conditions. For the animal studies, the mice were earmarked before grouping and were then randomly separated into groups by one person; however, no particular method of randomization was used. The sample size was predetermined empirically according to previous experience using the same strains and treatments. No animals were excluded from the analysis. Generally, the investigator was not blinded to the group allocation when assessing the outcome. We ensured that the experimental groups were balanced in terms of animal age and weight. All animal studies were approved by the Animal Experimentation Ethics Committee of the Chinese Academy of Medical Sciences (permit no. 002802), and all procedures were conducted in accordance with the guidelines of the Institutional Animal Care and Use Committees of the Chinese Academy of Medical Sciences. The animal study was also conducted in accordance with the Animal Research: Reporting of In vivo Experiments (ARRIVE) guidelines.
Statistical analysis
The Wilcoxon Mann-Whitney test was used to compare the distributions of numerical variables between patients with APL and patients with other types of AML. The associations between qualitative variables were assessed by the χ2 test. All statistics were computed using SPSS software, version 22.0. P values < 0.05 were considered statistically significant.
Results
APL patients have a higher prevalence of dyslipidemia than non-APL AML patients
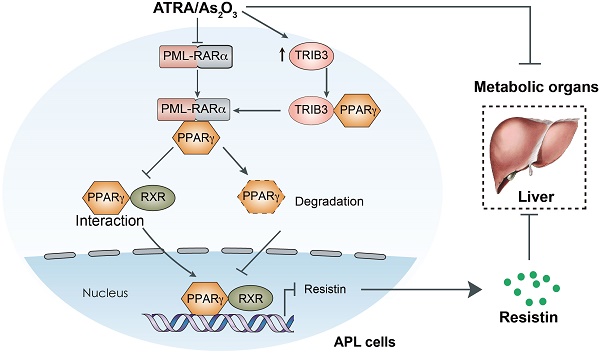
A retrospective study (Supplementary Table 1) was conducted to investigate lipid profiles and other major clinical parameters in 120 newly diagnosed AML patients (60 APL patients versus 60 non-APL AML patients). More patients were overweight (BMI > 25) in the APL group (52%, 31/60) than in the non-APL AML group (32%, 19/60) (p = 0.02). However, the obesity rate (BMI > 30) was not different between the APL and non-APL AML patients (13% vs. 5%, p = 0.11). Hyperlipidemia was found in 65% (39/60) of APL patients and in 36% (22/60) of non-APL patients (p = 0.0019). The initial levels of TGs before treatment were higher in the APL patients than in the non-APL patients (Figure 1A). Moreover, the TC, HDL and LDL levels in the APL patients were higher than those in the non-APL patients (Figure 1B-D), indicating that a higher proportion of APL patients had dyslipidemia.
To examine the effect of APL cells on dyslipidemia in vivo, normal FVB mice were transplanted with APL cells from hMRP8-Pml-Rarα mice, and the body/liver weight, liver lipid levels, and blood lipid levels of recipient mice were assessed over time (Figure 1E). The body and liver weights of mice transplanted with APL cells were higher than those of mice transplanted with normal spleen cells (Figure 1F-H). Additionally, the APL cell-transplanted mice showed elevated TG and cholesterol levels in the liver (Figure 1I). Moreover, the serum TC and TG levels in APL cell-transplanted mice were higher than those in normal spleen cell-transplanted mice (Figure 1J). To explore whether APL cells are the main inducers of dyslipidemia in vivo, we assessed the liver and serum TC levels in NOD scid gamma (NSG) mice transplanted with APL or non-APL C1498 AML cells (Figure 1K). Consistent with the clinical data, the liver and serum TC levels in NSG mice transplanted with APL cells were higher than those of C1498 cell-transplanted NSG mice over time after inoculation (Figure 1L-M). Overall, these data indicate that APL cells play a critical role in the enhancement of liver and serum lipid levels in both APL patients and mice.
Given that the lipid production capacity of leukemia cells is less than that of metabolic organs, such as the liver, we next examined the effects of APL cells on the lipid metabolism of hepatocytes (Figure 2A). We found that the triglyceride and cholesterol production in mouse primary hepatocytes was increased by APL cells derived from hMRP3-Pml-Rara mice but not by murine non-APL AML cells (Figure 2B). Simultaneously, APL cells promoted the secretion of the proprotein convertase subtilisin/kexin type 9 (PCSK9) in hepatocytes as indicated by ELISA (Figure 2C) and subsequently reduced low-density lipoprotein receptor (LDLR) expression in hepatocytes (Figure 2D). PML-RARα acts as the driver of genetic alteration and the most critical factor responsible for the pathogenesis of > 95% of APL cases. To investigate whether PML-RARα contributed to the dyslipidemia associated with APL, we examined the serum TG, HDL and LDL levels in myeloid cell-specific Pml-Rarα knockin (Pml-RarαKI) mice (Figure 2E). Serum TG, HDL, and LDL levels were higher in 3- to 4-month-old Pml-RarαKI mice (no APL symptoms) than in wild-type (WT) mice of the same age (Figure 2F-H), indicating that PML-RARα plays a critical role in the lipid metabolism disorder associated with APL.
Lipid profiles of patients with APL and mice transplanted with APL cells from hMRP8-Pml-Rarα APL mice. (A-D) Serum triglyceride (TG), total cholesterol (TC), high-density lipoprotein (HDL) and low-density lipoprotein (LDL) levels in APL patients (n = 60) and non-APL AML patients (n = 60) were evaluated before induction therapy. (E) Approaches to define the effects of murine APL cells or normal spleen cells on the body/liver weights, liver lipid levels, and blood lipid levels of FVB recipient mice. (F and G) Body weights (F) and liver weights (G) of the FVB recipient mice transplanted with normal spleen cells (CTRL) and Pml-Rarα APL cells (Pml-Rarα) at the indicated times after inoculation. (H) Representative liver morphologies of CTRL and Pml-Rarα recipient mice at 3 weeks after inoculation. (I) Total triglyceride (TG) and cholesterol levels of the liver lipids extracted using the chloroform/methanol method. The data were normalized to the liver weights and are represented as the mean ± the standard error (SEM). N = 3 mice per group. (J) Serum TG and total cholesterol (TC) levels in the CTRL and Pml-Rarα recipient FVB mice at the indicated times after inoculation. (K) Approaches to define the effects of murine APL cells or non-APL AML cells (C1498) on liver and blood TC levels in NSG recipient mice. (L and M) Liver and serum TC levels in recipient NSG mice transplanted with normal spleen cells (CTRL), Pml-Rarα APL cells, or C1498 AML cells at the indicated times after inoculation. For panels F-M, n = 4 mice per group. APL: acute promyelocytic leukemia; AML: acute myeloid leukemia; ULN: upper limits of normal.
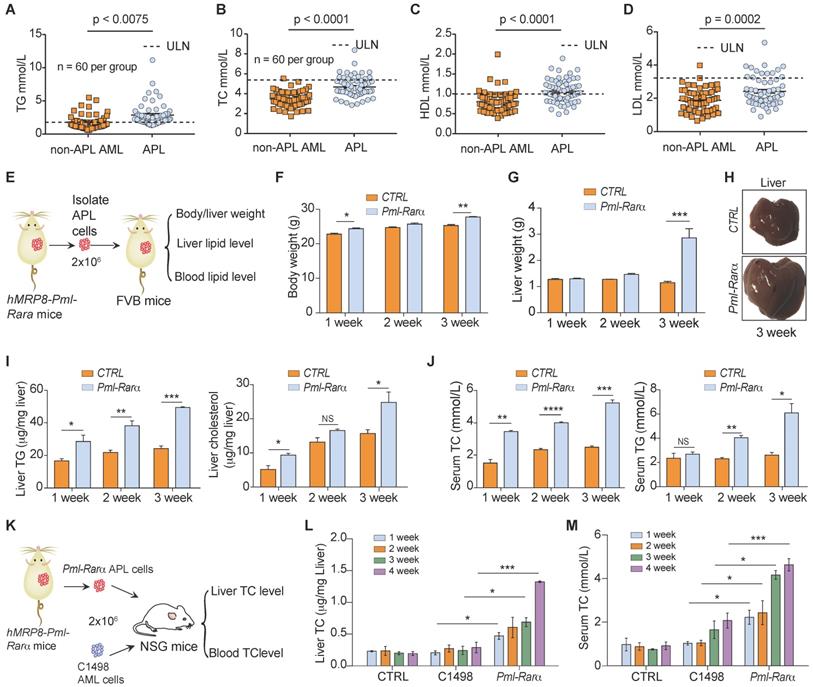
PML-RARα-positive APL cells induced lipid production in hepatocytes. (A) Approaches to define the effects of murine APL cells or C1498 AML cells on lipid production and LDLR expression in hepatocytes. (B) Total triglyceride (TG) and cholesterol levels in culture supernatants from mouse hepatocytes were determined by enzymatic assay. (C) The PCSK9 levels in the culture supernatants of mouse hepatocytes cocultured with the indicated cells were detected by ELISA. (D) Confocal assay of LDLR expression in mouse hepatocytes cocultured with C1498 AML or APL cells. (E) Approaches to define murine serum TG/HDL/LDL levels in myeloid cell-specific Pml-Rarα knockin (Pml-RarαKI) mice (3-4 months old). (F-H) Serum TG, HDL, and LDL levels in WT mice and Pml-RarαKI mice at the age of 3-4 months.
PML-RARα inhibits PPARγ transcriptional activity to induce dyslipidemia in APL patients
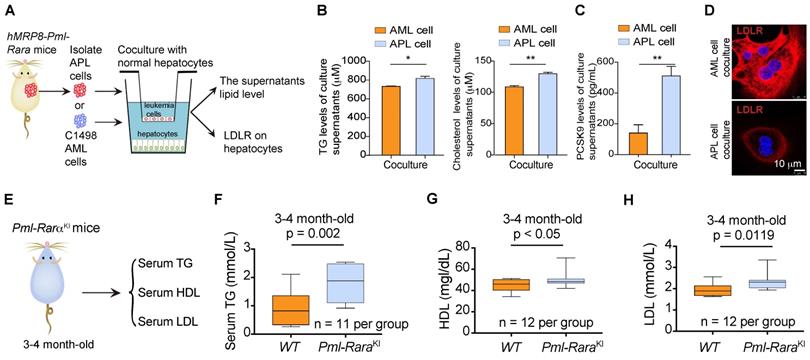
To investigate the mechanisms underlying the high prevalence of dyslipidemia in APL patients, we performed gene set enrichment analysis (GSEA) on differentially expressed genes (DEGs) enriched in APL and non-APL AML subtypes. Notably, the lipid metabolism process was identified as one of the most significantly enriched Kyoto Encyclopedia of Genes and Genomes (KEGG) gene sets associated with APL (Figure 3A). Moreover, several lipid metabolism-related genes (RETN, GPHN, ME1, LEP, LTC4S, DHCR7, TRIB3, MOSC2, ABCA1, and PPARG) ranked among the top dysregulated genes in APL patients versus non-APL AML patients. We further analyzed the individual expression of the lipid metabolism genes in these AML subtypes and found that the expression levels of only RETN, LEP, PPARG and TRIB3 were significantly dysregulated in APL cells compared with non-APL AML cells (Figure 3B, Figure S1A and Table S2). Leukemia cells from most APL patients showed reduced PPARγ protein expression and enhanced resistin and TRIB3 protein levels compared with those in leukemia cells from non-APL patients (Figure 3C). The adipokines leptin and resistin, transcriptionally regulated by PPARγ, are involved in the regulation of glucose and lipid metabolism. Serum resistin, but not leptin, was also dysregulated in APL patients (Figure 3D and Figure S1B). Furthermore, overexpression of PML-RARα decreased the protein level of PPARγ in non-APL U937 cells, accompanied by increased resistin and reduced leptin expression (Figure S1C-D). Thus, the serum resistin and PCSK9 levels in APL cell-transplanted FVB mice were higher than those in the control mice over time (Figure 3E-F). To verify whether the adipokine resistin secreted from APL cells could enhance lipid levels in hepatocytes, we treated normal liver cells with resistin (Figure 3G) and found that resistin stimulation lowered LDLR expression in hepatocytes and enhanced the levels of TG, TC and PCSK9 in the culture supernatants of hepatocytes (Figure 3H-I). Consistently, resistin-knockdown APL cells almost lost the ability to enhance the levels of TG, TC and PCSK9 in the culture supernatants of hepatocytes (Figure 3J-L). Collectively, these data indicate that APL cells elevate lipid levels in APL mice and that PML-RARα acts as the core factor causing the dyslipidemia associated with APL.
To determine how PML-RARα is involved in the metabolic disorders associated with APL, we examined the expression of PM-RARα and PPARγ in leukemia cells from APL patients. The expression of PML-RARα was negatively correlated with that of PPARγ in APL cells (Figure 4A). The transcriptional activity of PPARγ, but not PPARα, was inhibited by overexpression of PML-RARα in APL cells (Figure 4B). Indeed, adipose PPARγ is a well-known mediator of organism-wide metabolism [16]; however, it is still unclear whether myeloid PPARγ has similar effects. Given that APL cells have a low level of endogenous PPARγ protein, PPARγ expression was stably depleted in non-APL myeloid leukemia cells, and the effects of these cells on the liver and serum lipid levels were examined in the mice (Figure 4C). Non-APL myeloid leukemia cells induced no liver TG or TC enhancement over time in vivo, but PPARγ knockdown cells increased both the liver TG and TC levels of the transplanted mice (Figure 4D & E). Similarly, increases in serum TG and TC levels were observed in mice transplanted with PPARγ-depleted leukemia cells but not in mice transplanted with control leukemia cells (Figure 4D & E). Moreover, the lipid levels of mice transplanted with PPARγ-depleted leukemia cells were much higher than those of control mice (Figure 4D-G). These data indicate that altered PPARγ activity in APL cells is responsible for the dyslipidemia observed in APL mice.
PPARγ signaling is dysregulated in PML-RARα-positive cells. (A) Gene set enrichment analysis (GSEA) shows the enrichment of lipid metabolism process-related genes (GSE13204) in APL cells (n = 50) versus non-APL AML cells (n = 50). (B) qRT-PCR was performed to analyze the mRNA levels of RETN, LEP, PPARG, and TRIB3 (normalized to GAPDH) in primary APL cells and non-APL AML cells. (C left) The expression of PML-RARα, PPARγ, resistin, leptin, and TRIB3 was detected by Western blotting in primary APL cells and non-APL AML cells. (C right) Statistical analyses of PPARγ, resistin, leptin, and TRIB3 expression in primary APL cells (n = 16) and non-APL AML cells (n = 8). (D) Serum resistin levels in newly diagnosed APL patients (n = 34) and non-APL AML patients (n = 13). (E) Approaches to define the effects of murine APL cells or normal spleen cells on serum resistin and PCSK9 levels in FVB recipient mice. (F) Serum resistin (left) and PCSK9 (right) levels in FVB recipient mice transplanted with normal spleen cells (CTRL) and APL cells (Pml-Rarα) at the indicated times after inoculation (n = 4 per group). (G) Approaches to define the effects of resistin on the LDLR expression and lipid secretion of hepatocytes. (H) The LDLR expression in normal mouse hepatocytes was detected by confocal assay after resistin (50 ng/mL) stimulation for 24 h. (I) The levels of TG, cholesterol and PCSK9 in the supernatants of normal mouse hepatocytes after resistin (50 ng/mL) stimulation for 24 h. (J) Approaches to define the effects of resistin-depleted murine APL cells on the TG, cholesterol and PCSK9 secretion of hepatocytes. (K and L) The levels of TG, cholesterol and PCSK9 in the supernatants of normal mouse hepatocytes after coculture with APL cells with or without resistin depletion for 24 h.
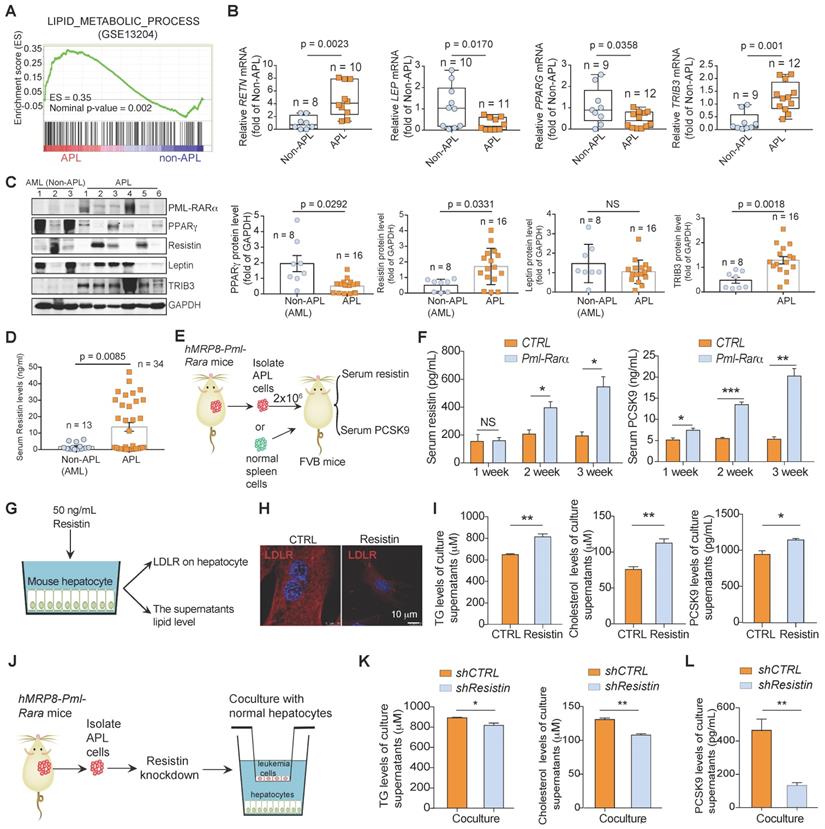
Reduced PPARγ activity in APL cells causes lipid metabolism disorder in APL patients. (A) Correlation between PML-RARα and PPARγ expression (protein level) in human primary APL cells (n = 16). Each data point represents the value from an individual patient. Statistical significance was measured by Pearson's correlation test. (B) PML-RARα decreased the transcriptional activity of PPARγ. HEK 293T cells were transiently transfected with the indicated plasmids. After 24 h of transfection, luciferase activities were measured. (C) Approaches to define the effects of C1498 AML cells with or without PPARγ depletion on the liver and blood lipid levels of C57 BL/6 recipient mice. (D-G) Liver and serum TG and TC levels in mice transplanted with CTRL shRNA C1498 cells or PPARγ shRNA C1498 cells at the indicated times after inoculation. For panel D-G, n = 4 mice per group.
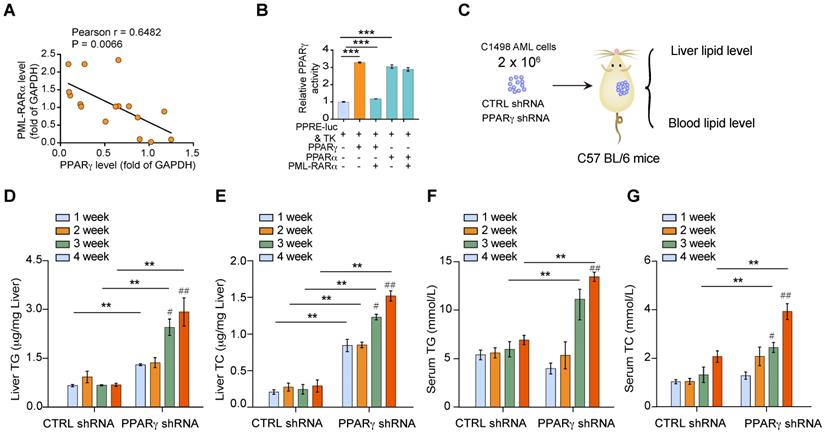
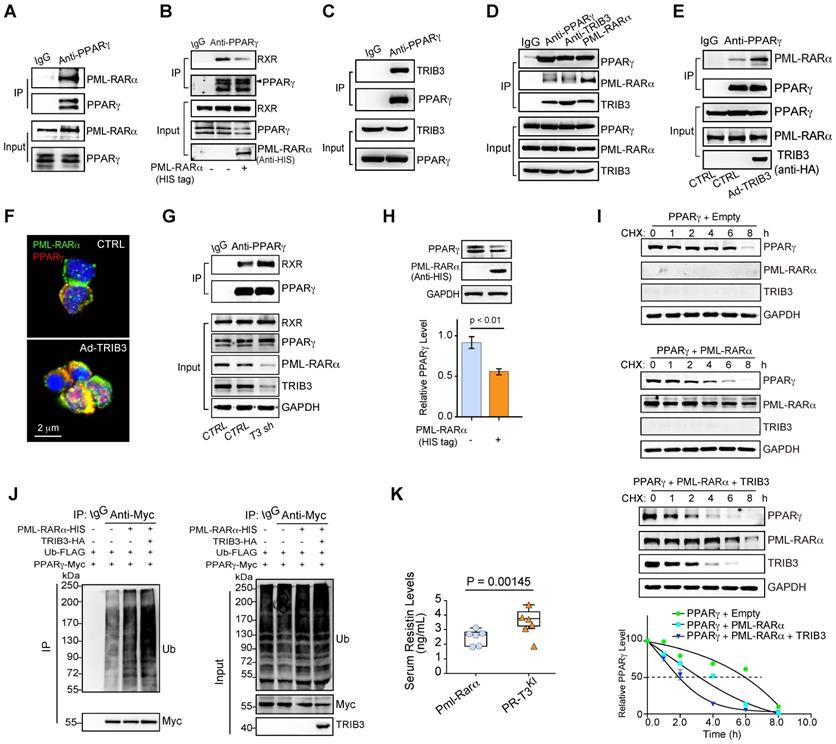
PPARγ is a ligand-activated transcription factor and functions as a heterodimer with an RXR [31]. Mechanistically, this finding was verified by the interaction of PML-RARα and PPARγ in human APL cells (Figure 5A). PML-RARα overexpression decreased the interaction of PPARγ and RXR in APL cells (Figure 5B). Elevated TRIB3 expression promotes lipid metabolism and sustains the oncogenic function of PML-RARα in APL patients via protein-protein interactions [23]. Indeed, TRIB3 coimmunoprecipitated with PPARγ in APL cells (Figure 5C). Furthermore, TRIB3, PPARγ and PML-RARα formed a heterotrimer (Figure 5D), and elevated TRIB3 promoted the binding of PML-RARα and PPARγ in APL cells (Figure 5E-F). Moreover, TRIB3 depletion rescued the interaction between PPARγ and RXR by decreasing PML-RARα expression (Figure 5G). These data indicate that the collaboration of PML-RARα and TRIB3 inhibits PPARγ activity by disrupting the PPARγ/RXR heterodimer.
Additionally, overexpression of PML-RARα decreased PPARγ protein expression (Figure 5H). We assessed the role of PML-RARα in the regulation of PPARγ ubiquitination and degradation mediated by ubiquitin ligases [32, 33]. In the presence of the protein synthesis inhibitor cycloheximide (CHX), the PPARγ protein exhibited a reduced half-life in PML-RARα-overexpressing cells compared with that in the control group. TRIB3 overexpression further accelerated the degradation of PPARγ mediated by PML-RARα overexpression (Figure 5I). PML-RARα overexpression increased the ubiquitination of PPARγ and TRIB3 further increased the ubiquitination of PPARγ mediated by PML-RARα overexpression (Figure 5J). Furthermore, Trib3 knockin Pml-Rarα (PR-T3KI) mice [23] showed elevated serum resistin levels compared with those in the Pml-RarαKI mice (Figure 5K). Given the interconnectivity between PML-RARA expression and PPARG/TRIB3, we investigated whether these genes are direct targets (transcriptionally) of PML-RARα by transfecting Myc-tagged PML-RARA plasmids into NB4 cells and used an anti-Myc antibody to capture protein-DNA complexes. The ChIP-qPCR results verified that both PPARG and TRIB3 are direct target genes of PML-RARA (Figure S2). Thus, these gain-of-function studies indicate crucial roles of PML-RARα and TRIB3 in the inhibition of PPARγ activity by interrupting the PPARγ/RXR heterodimer and promoting ubiquitination-dependent PPARγ degradation.
The combination of PML-RARα and TRIB3 inhibits PPARγ activity by disrupting the PPARγ/RXR heterodimer and promoting PPARγ degradation. (A) The interaction between endogenous PML-RARα and PPARγ was detected by a coimmunoprecipitation (co-IP) assay in human primary APL cells. (B) PML-RARα reduced the interaction between PPARγ and RXR. NB4 cells were transfected with or without a PML-RARα-expressing plasmid. After 24 h of transfection, co-IP analysis was performed to detect the PPARγ/RXR interaction. (C) The interaction between TRIB3 and PPARγ was detected by a co-IP assay in HEK 293T cells transfected with TRIB3- and PPARγ-expressing plasmids. (D) TRIB3, PML-RARα and PPARγ trimers were detected by a co-IP assay in HEK 293T cells. HEK 293T cells were transiently transfected with PPARγ-, TRIB3-, and PML-RARα-expressing plasmids. After 24 h of transfection, a co-IP assay was performed to detect the interactions between TRIB3, PML-RARα and PPARγ. (E) TRIB3 increased the interaction between PPARγ and PML-RARα. NB4 cells were infected with an adenovirus expressing TRIB3 or an HA tag (CTRL). After 24 h of transfection, a co-IP analysis was performed to detect the PPARγ/PML-RARα interaction. (F) TRIB3 increased the colocalization of PPARγ and PML-RARα. Human primary APL cells were infected with adenovirus expressing TRIB3 or CTRL. After 24 h of transfection, the colocalization of PPARγ/PML-RARα was detected by an IF staining assay. (G) TRIB3 depletion increased the interaction between PPARγ and RXR. A co-IP analysis was performed to detect the PPARγ/RXR interaction in NB4 cells with or without TRIB3 depletion. (H) The effects of PML-RARα on the protein level of PPARγ in U937 cells. (I) The effect of PML-RARα or PML-RARα and TRIB3 overexpression on PPARγ degradation. HEK 293T cells were transfected with the indicated plasmids, and 12 h later, the cells were incubated with CHX (10 μg/mL) for the indicated times. (J) The effect of PML-RARα overexpression or PML-RARα and TRIB3 overexpression on PPARγ ubiquitination. HEK 293T cells were transfected with the indicated plasmids, and 12 h later, cell extracts were IP with anti-Myc Ab. Ubiquitinated PPARγ was detected by immunoblotting. (K) Serum resistin levels in Pml-Rarα mice (nonleukemic) with or without Trib3 knockin (3 months old).
ATRA/arsenic rescues PPARγ expression in APL cells but does not ameliorate dyslipidemia in APL patients
ATRA plus arsenic trioxide (As2O3) with or without chemotherapy induces high remission rates in APL patients by degrading the oncoprotein PML-RARα [34, 35]. Given that PML-RARα inhibited PPARγ activity in APL cells, we next examined whether ATRA/As2O3 rescued PPARγ activity and improved dyslipidemia in APL patients by decreasing PML-RARα expression. The combination of ATRA and As2O3 enhanced PPARγ expression in leukemia cells from APL patients after 1 week of induction therapy (Figure 6A). Furthermore, As2O3 treatment rescued the colocalization of PPARγ and RXR by degrading the PML-RARα protein (Figure 6B). However, the mRNA and protein levels of resistin were enhanced, whereas the protein level of leptin was decreased in APL cells after As2O3 or ATRA treatment (Figure 6C & D), which was accompanied by elevated resistin secretion and downregulated leptin secretion (Figure 6E) in the culture supernatant of APL cells after As2O3 treatment.
ATRA/As2O3 treatment increases PPARγ expression but does not improve dyslipidemia. (A) ATRA/As2O3 treatment increased PPARγ expression in APL cells. The PPARγ expression in primary APL cells isolated from the bone marrow of APL patients was detected by Western blotting before treatment and 1 week after combined ATRA and arsenic treatment. (B) Colocalization of PPARγ (red) and RXR (green) in primary human APL cells (APL-1#) was detected by immunostaining. Scale bar, 10 μm. (C) qRT-PCR was performed to analyze the RETN mRNA levels in primary APL cells treated as indicated for 24 h. (D) The expression of leptin and resistin in primary APL cells was detected by Western blotting after administration of the indicated treatment for 24 h. (E) Effects of arsenic treatment on resistin and leptin secretion in primary APL cells after 2 days of treatment. (F) Approaches to define the effects of ATRA or ATRA and leptin on the lipid production of murine hepatocytes cocultured with murine APL cells. (G and H) The levels of TG (G) and cholesterol (H) in the supernatants of normal mouse hepatocytes after coculture with primary APL cells treated with ATRA or ATRA and leptin for 12 h. (I) ATRA and arsenic treatment increased serum TG levels in APL patients. The serum TG levels of APL patients were measured by Abbott ARCHITECT c16000 at the indicated treatment times (n = 60), ULN: upper limits of normal. (J) ATRA and arsenic treatment increased serum resistin levels in APL patients (n = 12).
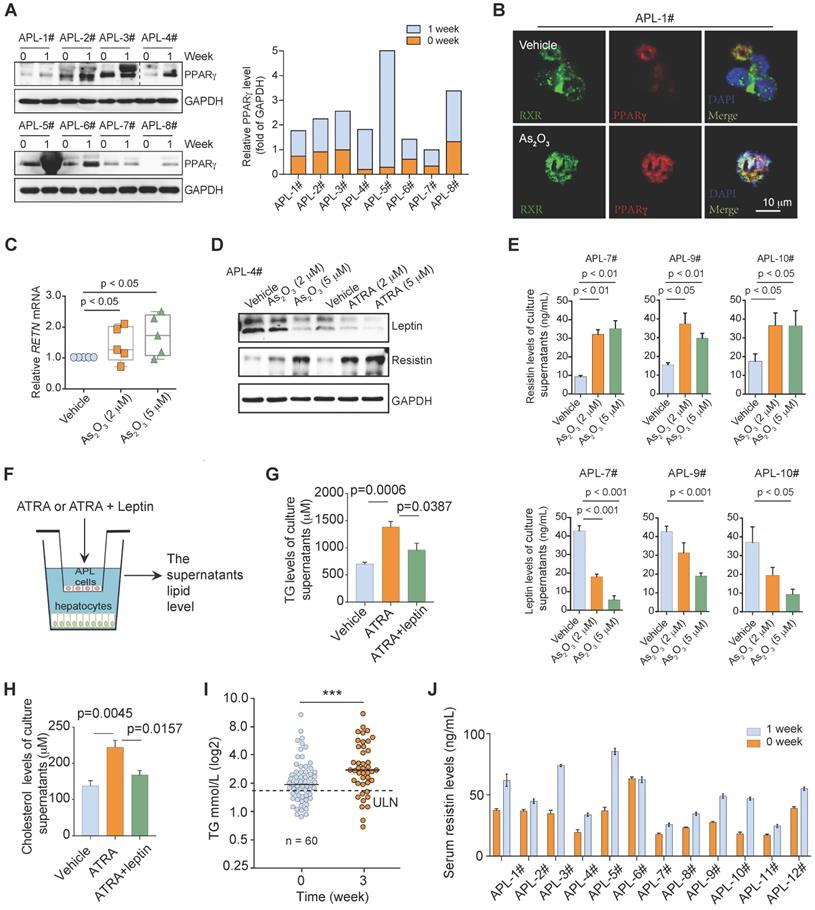
We next assessed the effects of leptin on the secreted TC and TG levels of the supernatant of hepatocytes cells cocultured with APL cells treated with ATRA (Figure 6F). Increased TC and TG levels were observed in the ATRA-treated coculture system of primary mouse APL cells and hepatocytes. Leptin partially protected against the ATRA-induced TC and TG enhancement of hepatocytes cocultured with APL cells (Figure 6G & H). These data indicated that decreased leptin contributed to the dyslipidemia phenotypes caused by ATRA treatment. We further analyzed the lipid profiles of APL patients during first-line therapy. The TG concentrations of APL patients increased after 3 weeks of induction therapy (Figure 6I). Consistently, the serum resistin levels of most APL patients were also elevated after 1 week of induction therapy (Figure 6J and Table S2). The observation that anti-APL therapy reduced PML-RARα and enhanced PPARγ but could not normalize dyslipidemia in APL patients indicates that other important regulator(s) may participate in the regulation of lipid metabolism disorder in APL patients during induction therapy.
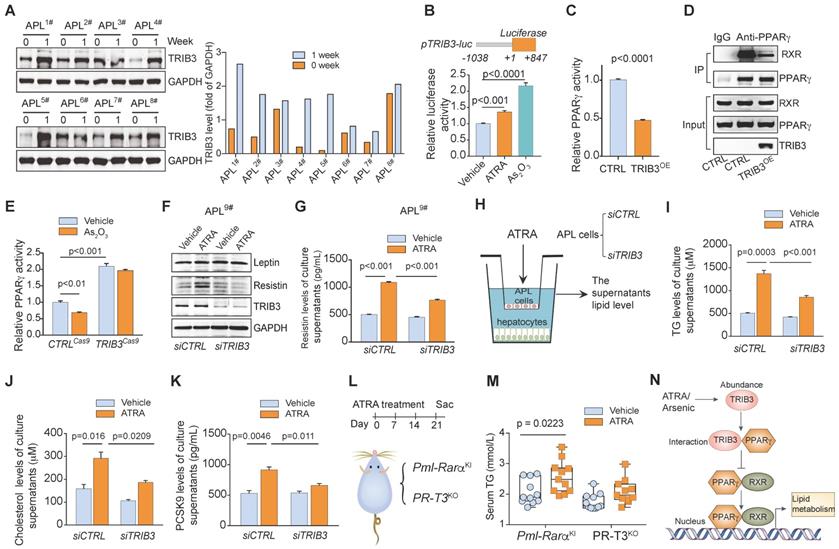
High TRIB3 mediates ATRA/arsenic therapy-induced dyslipidemia in APL patients
We found that combined ATRA and As2O3 therapy increased TRIB3 abundance in APL cells from most patients after 1 week of therapy (Figure 7A and Table S2). Furthermore, ATRA or As2O3 enhanced the transcription of TRIB3 in APL cells (Figure 7B). TRIB3 was reported to suppress adipocyte differentiation by negatively regulating PPARγ transcriptional activity [26]. Indeed, TRIB3 overexpression inhibited the transcriptional activity of PPARγ (Figure 7C) by interrupting the interaction between RXR and PPARγ (Figure 7D) in APL cells. TRIB3 deletion not only increased the transcriptional activity of PPARγ but also rescued the reduced PPARγ activity induced by As2O3 treatment (Figure 7E). Although TRIB3 depletion showed no effects on leptin expression in APL cells, silencing TRIB3 impeded the enhancement of resistin expression induced by ATRA treatment in APL cells (Figure 7F). Moreover, TRIB3 depletion ameliorated the elevated resistin production in the culture supernatants of APL cells treated with ATRA (Figure 7G). Additionally, we examined the effects of ATRA on the levels of TC, TG and PCSK9 in the supernatant of primary hepatocytes cocultured with mouse APL cells with or without TRIB3 depletion (Figure 7H). Loss of TRIB3 moderately counteracted the increased levels of TC, TG and PCSK9 secretion from hepatocytes cocultured with APL cells treated with ATRA (Figure 7I-K). Importantly, ATRA treatment induced increased serum TG levels in Pml-Rarα transgenic mice but did not elevate TG levels in Trib3-knockout Pml-Rarα transgenic mice (PR-T3KO) (Figure 7L & M), indicating that TRIB3 is involved in the regulation of anti-APL-therapy-induced dyslipidemia in individuals with APL. Overall, arsenic/ATRA treatment enhances TRIB3 abundance in APL cells, and TRIB3 interacts with PPARγ to impede the heterodimer formation of PPARγ and RXR, inhibiting PPARγ transcriptional activity and abnormal lipid metabolism in APL cells (Figure 7N).
Collaboration of PPAR agonists and ATRA/arsenic improves dyslipidemia in APL patients
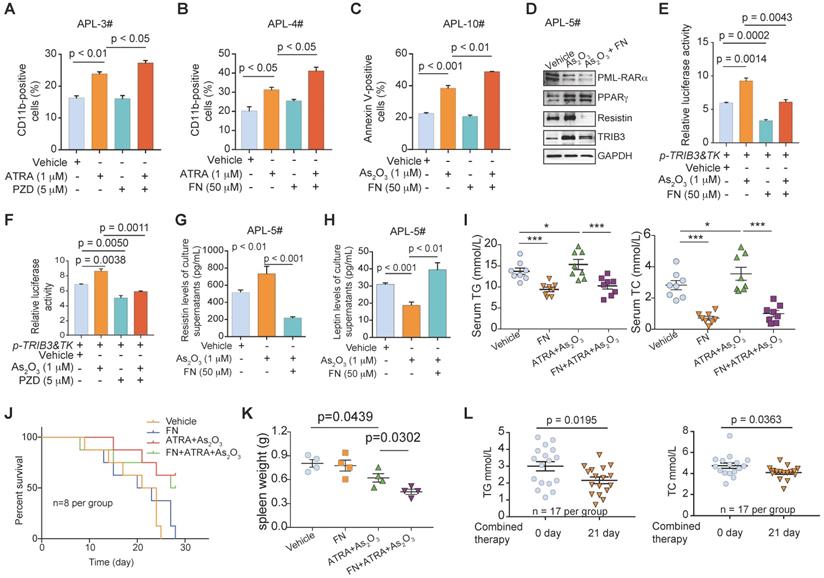
Given that APL patients had a high prevalence of dyslipidemia before and during induction therapy, the use of a lipid-lowering drug combined with ATRA and As2O3 therapy may further benefit APL patients and improve their clinical outcomes. First, we examined the effects of pharmacological PPAR activation in APL cells. A PPARγ activator (pioglitazone, PZD) synergized with ATRA-mediated differentiation in primary APL cells (Figure 8A). Similarly, the PPARα agonist fenofibrate (FN) potently increased the differentiation and apoptosis percentages of APL cells induced by ATRA or an arsenic agent (Figure 8B & C). Furthermore, the PPAR activator decreased PML-RARα expression and impeded the increased resistin induced by As2O3 treatment (Figure 8D). Interestingly, the arsenic-induced increase in TRIB3 expression was hindered by FN treatment (Figure 8D), and the PPAR activators reduced the increase in TRIB3 transcription induced by arsenic treatment, indicating that PPAR activators nonspecifically inhibit TRIB3 (Figure 8E & F). Accordingly, FN treatment protected against elevated resistin secretion and reduced leptin secretion in APL cells treated with As2O3 (Figure 8G & H).
We next evaluated the therapeutic effect of ATRA/arsenic and FN in combination in the PML-RARα mouse model. Treatment of PML-RARα APL mice with the combination for 3 weeks improved lipid metabolism disorder associated with APL, as indicated by reductions in the elevated serum TG and TC levels in ATRA/arsenic-treated APL mice (Figure 8I). Although the combination did not significantly improve the survival rate of APL mice compared with those treated with ATRA/arsenic alone (Figure 8J), the PPAR agonist FN synergized with ATRA/arsenic therapy to decrease spleen weights in APL mice (Figure 8K). Similarly, treatment of APL patients (n = 17) with the combination for 3 weeks improved the dyslipidemia associated with APL, as indicated by reductions in the elevated TG and TC levels of APL patients (Figure 8L). Overall, these data showed that combined therapy with ATRA/arsenic and a PPAR agonist is suggested for APL patients.
ATRA/As2O3-enhanced TRIB3 inhibits PPARγ activity and increases dyslipidemia in APL. (A) ATRA/As2O3 treatment induced TRIB3 abundance in APL cells. TRIB3 expression in primary APL cells isolated from the bone marrow of APL patients (n = 8) was detected by Western blotting before treatment and 1 week after combined ATRA and arsenic treatment. (B) ATRA/arsenic treatment inhibited TRIB3 transcription. HEK 293T cells were transiently transfected with the pTRIB3-luc and TK plasmids. After 24 h of transfection, cells were treated with ATRA or arsenic for 24 h, and luciferase activities were measured. (C) TRIB3 decreased the transcriptional activity of PPARγ. HEK 293T cells were transiently transfected with PPRE-Luc, TK, and CTRL or TRIB3 overexpression plasmids. After 24 h of transfection, the cells were treated with PZD (5 μM), and luciferase activities were measured. (D) TRIB3 reduced the interaction between PPARγ and RXR. NB4 cells were transfected with or without a TRIB3-expressing plasmid. After 24 h of transfection, co-IP analysis was performed to detect the PPARγ/RXR interaction. (E) The effect of As2O3 treatment on the transcriptional activity of PPARγ in NB4 cells with or without TRIB3 deletion. CTRLCas9 and TRIB3cas9 NB4 cells were transfected with PPARγ reporter genes. After 24 h of transfection, the cells were treated with vehicle or As2O3 for 24 h, and luciferase activities were measured. (F) The effect of ATRA treatment on the protein levels of leptin and resistin in primary APL cells with or without TRIB3 depletion. (G) The effect of ATRA treatment on the resistin level of culture supernatants of primary APL cells with or without TRIB3 depletion. (H) Approaches to define the effects of TRIB3-depleted murine APL cells on the TG, cholesterol and PCSK9 secretion of hepatocytes. (I-K) The levels of TG (I), cholesterol (J) and PCSK9 (K) in the supernatants of normal mouse hepatocytes after coculture with TRIB3-depleted APL cells treated with ATRA for 12 h. (L) Strategy for studying the effects of ATRA treatment on the serum TG levels in Pml-Rarα (Pml-RarαKI) and Trib3 knockout Pml-Rarα (PR-T3KO) mice. (M) Serum TG levels in the indicated mice treated with or without ATRA (8 months old). (N) Schematic diagram illustrating the role of TRIB3 in the regulation of PPARγ activity in APL cells treated with ATRA/arsenic.
In summary, our study suggests that the collaboration of PML-RARα with elevated TRIB3 expression inhibits PPARγ activity and causes lipid metabolism abnormalities in newly diagnosed APL patients. The TRIB3 expression that was increased by ATRA/As2O3 treatment further impeded PPARγ activity by forming the heterotrimer of TRIB3, PML-RARα and PPARγ, contributing to dyslipidemia in APL patients undergoing anti-APL therapy.
Discussion
Previous studies demonstrated that TRIB3 interaction with PPARγ negatively regulates PPARγ activity in adipose tissue [26]. Our recent work indicates that elevated TRIB3 expression stabilizes the oncoproteins PML-RARα and PML in APL [23, 24] and that PML exerts its essential role in breast cancer cell and hepatic stellate cell (HSC) maintenance through regulation of PPAR signaling and fatty acid oxidation (FAO) [36, 37]. However, we do not know whether or how TRIB3, PPARγ and PML-RARα contribute collaboratively to the regulation of lipid metabolism in APL cells. In this study, we verified the hypothesis that the metabolic stress sensor TRIB3 collaborates with the oncoprotein PML-RARα to inhibit PPARγ activity and subsequently cause dyslipidemia in newly diagnosed APL patients. Moreover, elevated TRIB3 expression in response to arsenic/ATRA therapy further suppressed PPARγ activity by disrupting the PPARγ/RXR dimer, which contributes to abnormal lipid metabolism in arsenic/ATRA-treated APL patients. Accordingly, the PPAR activator not only enhanced the anti-APL effects of arsenic/ATRA in vitro but also reduced arsenic/ATRA-induced dyslipidemia in APL patients. Thus, our study may provide a rationale for the combination of ATRA/arsenic therapy with the PPAR activator for the treatment of patients with APL.
PPAR agonists induce the synergism of anti-APL with ATRA/As2O3 by improving dyslipidemia. (A) CD11b expression was evaluated by flow cytometry in primary APL cells that were subjected to the indicated treatment. The percentage of CD11b-positive cells was calculated with FCS Express software. The data are presented as the mean ± SEM of 3 assays. (B and C) The PPARα agonist (fenofibrate, FN) increased the percentage of differentiated and apoptotic APL cells induced by ATRA or As2O3 treatment. Primary APL cells were treated with the indicated treatment for 24 h and then stained with a CD11b antibody or Annexin-V‑PI and evaluated by flow cytometry. The data are presented as the mean ± SEM of 3 assays. (D) The expression of PML-RARα, PPARγ, TRIB3, and resistin in primary APL cells treated as indicated was detected by Western blotting. (E and F) PPAR agonists suppressed the elevated TRIB3 transcription induced by As2O3. HEK 293T cells were transiently transfected with the TRIB3 reporter plasmids (p-TRIB3&TK). After 24 h of transfection, the cells were treated with the indicated treatment for 24 h, and luciferase activities were then measured. (G and H) The PPARα agonist (FN) enhanced the secreted leptin level and reduced the resistin level in primary APL cells that were subjected to As2O3 treatment. The data are presented as the mean ± SEM of 3 assays. (I) Serum TG and TC levels in Pml-Rarα recipient FVB mice treated as indicated. (J) Kaplan-Meier survival curves for the Pml-Rarα recipient FVB mice treated as indicated (n = 8). (K) The data indicate the spleen weights of the Pml-Rarα recipient FVB mice treated as indicated. (L) Serum TG and TC levels in APL patients (n = 17) were evaluated after combined treatment with ATRA/arsenic plus FN.
Several studies have shown that APL patients have a higher percentage of obesity [38], which is considered an adverse prognostic indicator for clinical outcome in APL. However, the mechanism of the association between APL and obesity or overweight remains unclear. Less is known regarding the lipid profile statuses of newly diagnosed APL patients, although dyslipidemia has been associated with several types of cancer [39-41]. In this study, we showed that newly diagnosed APL patients indeed had a higher prevalence of dyslipidemia than non-APL AML patients, indicated by elevated serum levels of TC, TG, HDL and LDL. PPARγ is a transcription factor that plays a key role in adipogenesis and insulin sensitization, and polymorphisms in PPARγ have been associated with obesity and diabetes-related phenotypes, such as hyperinsulinemia and dyslipidemia [42, 43]. Here, we identified that APL cells with defective PPARγ function is likely the essential factor triggering the dyslipidemia of APL patients by secreting resistin and subsequently disrupting the lipid metabolism of hepatocytes. Although Jansen et al provided a preliminary clue that PML-RARα interferes with PPAR signaling pathways [19], little is known regarding the role and mechanism of PML-RARα in the lipid metabolism modulated by PPARγ. Based on these observations, our study revealed that PML-RARα interacts with PPARγ to disrupt the PPARγ/RXR heterodimer and promote PPARγ ubiquitination and degradation, which may partially explain the obesity and dyslipidemia in newly diagnosed patients with APL that is driven by PML-RARα. A recent study reported that the cytokine galectin-12, a negative regulator of lipolysis, is selectively overexpressed in APL cells [44] and may participate in lipid metabolism regulation in APL patients via lipid droplet accumulation. Our study showed that the production and secretion of the adipokines resistin and leptin were dysregulated in APL cells. Thus, galectin-12, resistin, leptin, and other adipokines/cytokines regulated by PPARγ may act synergistically to affect lipid metabolism in metabolic tissues, contributing to the dyslipidemia associated with APL.
The application of ATRA/arsenic treatment in APL makes APL a curable chronic disease, a great victory in the war against cancer in humans. However, although ATRA/arsenic treatment degraded PML-RARα and restored PPARγ expression, it did not improve but rather exacerbated dyslipidemia in these APL patients. One major reason is that retinoids induce hypertriglyceridemia in APL patients treated with ATRA. Several studies have indicated that the increased synthesis of cholesterol and TGs, the disproportion of apoprotein constituents in the liver, and defects in very-low-density lipoprotein (VLDL) clearance in skeletal muscle contribute to ATRA-induced hypertriglyceridemia in APL [45-47]. However, the detailed molecular mechanism of therapy-related dyslipidemia in APL has not been clear until now. Pseudokinase TRIB3, a critical modulator of glucose/lipid metabolism and cancer progression [48-50], has been reported to inhibit PPARγ activity in adipocytes via its interaction with PPARγ [26]. Although we previously found that TRIB3 expression is enhanced in APL cells [23] and arsenic enhances the mRNA and protein expression of TRIB3 [51], it is still unclear whether TRIB3 plays a role in the abnormal lipid level of treated APL patients. Our study revealed that TRIB3 induced by ATRA/arsenic therapy further inhibits PPARγ activity to dysregulate TG and adipocytokine secretion in APL cells, which subsequently contributes to the disordered lipid metabolism associated with APL. Thus, the inhibition of TRIB3 combined with ATRA/arsenic treatment may improve the lipid metabolism of APL patients. Interestingly, we found that PPAR agonists can reduce arsenic/ATRA-induced dyslipidemia in APL patients and decrease TRIB3 expression in APL cells. This effect may derive from the fact that PPAR agonists inhibit TGFβ1/Smad3 signaling [52] and that TRIB3 is a target gene of TGFβ1/Smad3 [53]. Thus, the beneficial effects of PPAR agonists in APL may be derived from not only the direct lowering of blood lipids but also a potential inhibition of TRIB3 expression; this concept needs further clarification.
In summary, our study not only reveals a critical role of the PML-RARα/PPARγ/TRIB3 axis in dyslipidemia for patients with APL but also confers a rationale for the combination of ATRA/arsenic with the PPAR activator for the treatment of patients with APL.
Abbreviations
APL: acute promyelocytic leukemia; ATRA: all-trans retinoic acid; AML: acute myelogenous leukemia; BMI: body mass index; DEGs: differentially expressed genes; ER: endoplasmic reticulum; FN: fenofibrate; GSEA: gene set enrichment analysis; HDL: high-density lipoprotein; KEGG: kyoto encyclopedia of genes and genomes; LDL: low-density lipoprotein; PPARγ: peroxisome proliferator-activated receptor-γ; PPRE: PPAR-response element; PZD: pioglitazone; RARs: Retinoic acid receptors; TRIB3: tribbles homologue 3; TC: total cholesterol; TG: triglyceride.
Supplementary Material
Supplementary figures and tables.
Acknowledgements
This work was supported by grants from the National Key R&D Program of China (2017YFA0205400), the National Natural Science Foundation of China (81530093, 81773781 to ZWH; 81570128 and 81970133 to HHZ; and 81872904 to KL), Beijing Outstanding Young Scientist Program (BJJWZYJH01201910023028), the CAMS Innovation Fund for Medical Sciences (2016-I2M-1-007 to ZHW; 2016-I2M-1-011 to KL; and 2016-I2M-3-008 to FW), and the “Ten thousand plan" - National high-level talents special support plan to KL.
Competing Interests
The authors have declared that no competing interest exists.
References
1. de Thé H, Pandolfi PP, Chen Z. Acute Promyelocytic Leukemia: A Paradigm for Oncoprotein-Targeted Cure. Cancer Cell. 2017;32(5):552-560
2. Breccia M, Mazzarella L, Bagnardi V. et al. Increased BMI correlates with higher risk of disease relapse and differentiation syndrome in patients with acute promyelocytic leukemia treated with the AIDA protocols. Blood. 2012;119(1):49-54
3. Estey E, Thall P, Kantarjian H, Pierce S, Kornblau S, Keating M. Association between increased body mass index and a diagnosis of acute promyelocytic leukemia in patients with acute myeloid leukemia. Leukemia. 1997;11(10):1661-1664
4. Jeddi R, Ghédira H, Mnif S, Gouider E, Fenaux P, Meddeb B. High body mass index is an independent predictor of differentiation syndrome in patients with acute promyelocytic leukemia. Leuk Res. 2010;34(4):545-547
5. Li S, Chen L, Jin W. et al. Influence of body mass index on incidence and prognosis of acute myeloid leukemia and acute promyelocytic leukemia: A meta-analysis. Sci Rep. 2017;7(1):17998
6. Tedesco J, Qualtieri J, Head D, Savani BN, Reddy N. High Prevalence of Obesity in Acute Promyelocytic Leukemia (APL): Implications for Differentiating Agents in APL and Metabolic Syndrome. Ther Adv Hematol. 2011;2(3):141-145
7. Zhu HH, Wu DP, Du X. et al. Oral arsenic plus retinoic acid versus intravenous arsenic plus retinoic acid for non-high-risk acute promyelocytic leukaemia: a non-inferiority, randomised phase 3 trial. Lancet Oncol. 2018;19(7):871-879
8. Lo-Coco F, Cicconi L. Towards home-based treatment for acute promyelocytic leukaemia, with caution. Lancet Oncol. 2018;19(7):846-847
9. Platzbecker U, Avvisati G, Cicconi L. et al. Improved Outcomes With Retinoic Acid and Arsenic Trioxide Compared With Retinoic Acid and Chemotherapy in Non-High-Risk Acute Promyelocytic Leukemia: Final Results of the Randomized Italian-German APL0406 Trial. J Clin Oncol. 2017;35(6):605-612
10. Bonet ML, Ribot J, Palou A. Lipid metabolism in mammalian tissues and its control by retinoic acid. Biochim Biophys Acta. 2012;1821(1):177-189
11. Hoshino T, Hatsumi N, Takada S, Sakura T, Miyawaki S. All-trans-retinoic acid as a possible cause of acute pancreatitis even in the absence of hypertriglyceridemia. Int J Hematol. 2008;88(1):121-122
12. Abou Chacra L, Ghosn M, Ghayad E, Honein K. A case of pancreatitis associated with all-trans-retinoic acid therapy in acute promyelocytic leukemia. Hematol J. 2001;2(6):406-407
13. Warrell RP Jr, de Thé H, Wang ZY, Degos L. Acute promyelocytic leukemia. N Engl J Med. 1993;329(3):177-189
14. Huang S, Wang LL, Xue NN, Li C, Guo HH, Ren TK. et al. Chlorogenic acid effectively treats cancers through induction of cancer cell differentiation. Theranostics. 2019;9(23):6745-6763
15. Gu W, Hu S, He B, Qiu G, Ma J, Chen Z. Metabolites of acute promyelocytic leukemia cells participate in contributing to hypertriglyceridemia induced by all-trans retinoic acid. Leuk Res. 2009;33(4):592-594
16. Evans RM, Barish GD, Wang YX. PPARs and the complex journey to obesity. Nat Med. 2004;10(4):355-361
17. Altucci L, Leibowitz MD, Ogilvie KM, de Lera AR, Gronemeyer H. RAR and RXR modulation in cancer and metabolic disease. Nat Rev Drug Discov. 2007;6(10):793-810
18. Zhu J, Nasr R, Pérès L. et al. RXR is an essential component of the oncogenic PML/RARA complex in vivo. Cancer Cell. 2007;12(1):23-35
19. Jansen JH, Mahfoudi A, Rambaud S, Lavau C, Wahli W, Dejean A. Multimeric complexes of the PML-retinoic acid receptor alpha fusion protein in acute promyelocytic leukemia cells and interference with retinoid and peroxisome-proliferator signaling pathways. Proc Natl Acad Sci U S A. 1995;92(16):7401-7405
20. Avery J, Etzion S, DeBosch BJ. et al. TRB3 function in cardiac endoplasmic reticulum stress. Circ Res. 2010;106(9):1516-1523
21. Du K, Ding J. Insulin regulates TRB3 and other stress-responsive gene expression through induction of C/EBPbeta. Mol Endocrinol. 2009;23(4):475-485
22. Liew CW, Bochenski J, Kawamori D. et al. The pseudokinase tribbles homolog 3 interacts with ATF4 to negatively regulate insulin exocytosis in human and mouse beta cells. J Clin Invest. 2010;120(8):2876-2888
23. Li K, Wang F, Cao WB. et al. TRIB3 Promotes APL Progression through Stabilization of the Oncoprotein PML-RARα and Inhibition of p53-Mediated Senescence. Cancer Cell. 2017;31(5):697-710.e7
24. Li K, Wang F, Hu ZW. Targeting pseudokinase TRIB3 brings about a new therapeutic option for acute promyelocytic leukemia. Mol Cell Oncol. 2017;4(4):e1337547
25. Qi L, Heredia JE, Altarejos JY. et al. TRB3 links the E3 ubiquitin ligase COP1 to lipid metabolism. Science. 2006;312(5781):1763-1766
26. Takahashi Y, Ohoka N, Hayashi H, Sato R. TRB3 suppresses adipocyte differentiation by negatively regulating PPARgamma transcriptional activity. J Lipid Res. 2008;49(4):880-892
27. Du K, Herzig S, Kulkarni RN, Montminy M. TRB3: a tribbles homolog that inhibits Akt/PKB activation by insulin in liver. Science. 2003;300(5625):1574-1577
28. O'Donnell MR, Tallman MS, Abboud CN. et al. Acute myeloid leukemia, version 2.2013. J Natl Compr Canc Netw. 2013;11(9):1047-1055
29. Li S, Ma Y, Tan Y. et al. Profiling and functional analysis of circular RNAs in acute promyelocytic leukemia and their dynamic regulation during all-trans retinoic acid treatment. Cell Death Dis. 2018;9(6):651
30. Liu X, Chen J, Yu S. et al. All-trans retinoic acid and arsenic trioxide fail to derepress the monocytic differentiation driver Irf8 in acute promyelocytic leukemia cells. Cell Death Dis. 2017;8(5):e2782
31. Chandra V, Huang P, Hamuro Y. et al. Structure of the intact PPAR-gamma-RXR- nuclear receptor complex on DNA. Nature. 2008;456(7220):350-356
32. Floyd ZE, Wang ZQ, Kilroy G, Cefalu WT. Modulation of peroxisome proliferator-activated receptor gamma stability and transcriptional activity in adipocytes by resveratrol. Metabolism. 2008;57(7):S32-S38
33. van Beekum O, Fleskens V, Kalkhoven E. Posttranslational modifications of PPAR-gamma: fine-tuning the metabolic master regulator. Obesity. 2009;17(2):213-219
34. Isakson P, Bjørås M, Bøe SO, Simonsen A. Autophagy contributes to therapy-induced degradation of the PML/RARA oncoprotein. Blood. 2010;116(13):2324-2331
35. Zhu J, Gianni M, Kopf E. et al. Retinoic acid induces proteasome-dependent degradation of retinoic acid receptor alpha (RARalpha) and oncogenic RARalpha fusion proteins. Proc Natl Acad Sci U S A. 1999;96(26):14807-14812
36. Carracedo A, Weiss D, Leliaert AK. et al. A metabolic prosurvival role for PML in breast cancer. J Clin Invest. 2012;122(9):3088-3100
37. Ito K, Carracedo A, Weiss D. et al. A PML-PPAR-δ pathway for fatty acid oxidation regulates hematopoietic stem cell maintenance. Nat Med. 2012;18(9):1350-1358
38. Castillo JJ, Mulkey F, Geyer S. et al. Relationship between obesity and clinical outcome in adults with acute myeloid leukemia: A pooled analysis from four CALGB (alliance) clinical trials. Am J Hematol. 2016;91(2):199-204
39. Fiorenza AM, Branchi A, Sommariva D. Serum lipoprotein profile in patients with cancer. A comparison with non-cancer subjects. Int J Clin Lab Res. 2000;30(3):141-145
40. Kuliszkiewicz-Janus M, Małecki R, Mohamed AS. Lipid changes occuring in the course of hematological cancers. Cell Mol Biol Lett. 2008;13(3):465-474
41. Naik PP, Ghadge MS, Raste AS. Lipid profile in leukemia and Hodgkin's disease. Indian J Clin Biochem. 2006;21(2):100-102
42. Osinski V, Bauknight DK, Dasa SSK. et al. In vivo liposomal delivery of PPARα/γ dual agonist tesaglitazar in a model of obesity enriches macrophage targeting and limits liver and kidney drug effects. Theranostics. 2020;10(2):585-601
43. Moon MK, Cho YM, Jung HS. et al. Genetic polymorphisms in peroxisome proliferator-activated receptor gamma are associated with Type 2 diabetes mellitus and obesity in the Korean population. Diabet Med. 2005;22(9):1161-1166
44. Xue H, Yang RY, Tai G, Liu FT. Galectin-12 inhibits granulocytic differentiation of human NB4 promyelocytic leukemia cells while promoting lipogenesis. J Leukoc Biol. 2016;100(4):657-664
45. Lalloyer F, Pedersen TA, Gross B. et al. Rexinoid bexarotene modulates triglyceride but not cholesterol metabolism via gene-specific permissivity of the RXR/LXR heterodimer in the liver. Arterioscler Thromb Vasc Biol. 2009;29(10):1488-1495
46. Gerber LE, Erdman JW Jr. Changes in lipid metabolism during retinoid administration. J Am Acad Dermatol. 1982;6(4):664-674
47. Davies PJ, Berry SA, Shipley GL. et al. Metabolic effects of rexinoids: tissue-specific regulation of lipoprotein lipase activity. Mol Pharmacol. 2001;59(2):170-176
48. Hua F, Li K, Yu JJ. et al. TRB3 links insulin/IGF to tumour promotion by interacting with p62 and impeding autophagic/proteasomal degradations. Nat Commun. 2015;6:7951
49. Li K, Zhang TT, Hua F, Hu ZW. Metformin reduces TRIB3 expression and restores autophagy flux: an alternative antitumor action. Autophagy. 2018;14(7):1278-1279
50. Li K, Zhang TT, Wang F. et al. Metformin suppresses melanoma progression by inhibiting KAT5-mediated SMAD3 acetylation, transcriptional activity and TRIB3 expression. Oncogene. 2018;37(22):2967-2981
51. Örd D, Örd T, Biene T, Örd T. TRIB3 increases cell resistance to arsenite toxicity by limiting the expression of the glutathione-degrading enzyme CHAC1. Biochim Biophys Acta. 2016;1863(11):2668-2680
52. Zhao C, Chen W, Yang L, Chen L, Stimpson SA, Diehl AM. PPARgamma agonists prevent TGFbeta1/Smad3-signaling in human hepatic stellate cells. Biochem Biophys Res Commun. 2006;350(2):385-391
53. Hua F, Mu R, Liu J. et al. TRB3 interacts with SMAD3 promoting tumor cell migration and invasion. J Cell Sci. 2011;124(19):3235-3246
Author contact
![]() Corresponding authors: E-mail: zhuhhdoccom (H.Z); huzhuoweiedu.cn (Z.H).
Corresponding authors: E-mail: zhuhhdoccom (H.Z); huzhuoweiedu.cn (Z.H).
 Global reach, higher impact
Global reach, higher impact