13.3
Impact Factor
Theranostics 2020; 10(3):968-997. doi:10.7150/thno.37215 This issue Cite
Review
Improving nanotherapy delivery and action through image-guided systems pharmacology
1. Center for Systems Biology, Massachusetts General Hospital, Boston, MA 02114.
2. Department of Radiology, Brigham and Women's Hospital and Harvard Medical School, Boston, MA 02115
3. Department of Radiology, Massachusetts General Hospital and Harvard Medical School, Boston, MA 02114.
4. Department of Systems Biology, Harvard Medical School, Boston, MA 02115.
Abstract
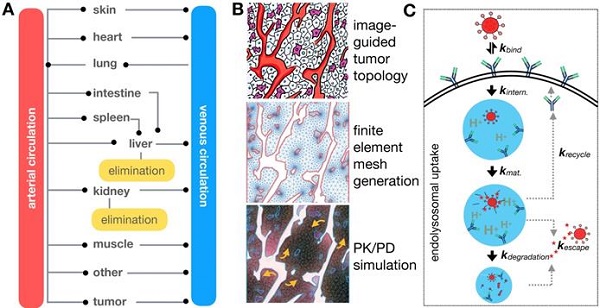
Despite recent advances in the translation of therapeutic nanoparticles (TNPs) into the clinic, the field continues to face challenges in predictably and selectively delivering nanomaterials for the treatment of solid cancers. The concept of enhanced permeability and retention (EPR) has been coined as a convenient but simplistic descriptor of high TNP accumulation in some tumors. However, in practice EPR represents a number of physiological variables rather than a single one (including dysfunctional vasculature, compromised lymphatics and recruited host cells, among other aspects of the tumor microenvironment) — each of which can be highly heterogenous within a given tumor, patient and across patients. Therefore, a clear need exists to dissect the specific biophysical factors underlying the EPR effect, to formulate better TNP designs, and to identify patients with high-EPR tumors who are likely to respond to TNP. The overall pharmacology of TNP is governed by an interconnected set of spatially defined and dynamic processes that benefit from a systems-level quantitative approach, and insights into the physiology have profited from the marriage between in vivo imaging and quantitative systems pharmacology (QSP) methodologies. In this article, we review recent developments pertinent to image-guided systems pharmacology of nanomedicines in oncology. We first discuss recent developments of quantitative imaging technologies that enable analysis of nanomaterial pharmacology at multiple spatiotemporal scales, and then examine reports that have adopted these imaging technologies to guide QSP approaches. In particular, we focus on studies that have integrated multi-scale imaging with computational modeling to derive insights about the EPR effect, as well as studies that have used modeling to guide the manipulation of the EPR effect and other aspects of the tumor microenvironment for improving TNP action. We anticipate that the synergistic combination of imaging with systems-level computational methods for effective clinical translation of TNPs will only grow in relevance as technologies increase in resolution, multiplexing capability, and in the ability to examine heterogeneous behaviors at the single-cell level.
Keywords: Intravital microscopy, Magnetic resonance imaging (MRI), Positron emission tomography / computed tomography (PET/CT), Pharmacokinetics / pharmacodynamics, Tumor microenvironment, Enhanced permeability and retention effect (EPR effect), Nanomedicine
 Global reach, higher impact
Global reach, higher impact