13.3
Impact Factor
Theranostics 2020; 10(2):880-897. doi:10.7150/thno.37178 This issue Cite
Research Paper
High-resolution 3D visualization of nanomedicine distribution in tumors
1. Bioscience, Discovery, Oncology R&D, AstraZeneca, Cambridge, United Kingdom;
2. Personalised Healthcare and Biomarkers, AstraZeneca, Macclesfield, United Kingdom;
3. Clinical Pharmacology and Safety Sciences, BioPharmaceuticals R&D, AstraZeneca, Cambridge, United Kingdom;
4. Department of Pharmaceutics, Utrecht University, Utrecht 3584 CG, The Netherlands; Department of Targeted Therapeutics, University of Twente, Enschede 7500 AE, The Netherlands;
5. Department of Nanomedicine and Theranostics, Institute for Experimental Molecular Imaging, RWTH Aachen University, Aachen 52074, Germany; Department of Pharmaceutics, Utrecht University, Utrecht 3584 CG, The Netherlands; Department of Targeted Therapeutics, University of Twente, Enschede 7500 AE, The Netherlands;
6. Pharmaceutical Sciences, BioPharmaceuticals R&D, AstraZeneca, Cambridge, United Kingdom;
7. Pharmaceutical Sciences, BioPharmaceuticals R&D, AstraZeneca, Macclesfield, United Kingdom.
*Present address: Medicines Discovery Catapult, Alderley Park, United Kingdom
Abstract
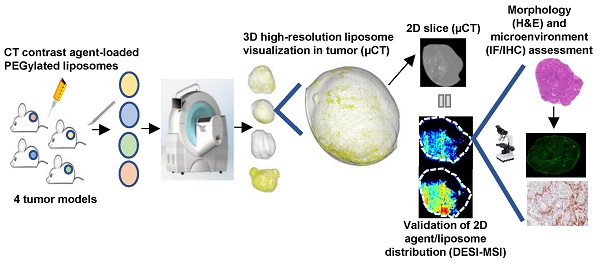
To improve the clinical translation of anti-cancer nanomedicines, it is necessary to begin building specific insights into the broad concept of the Enhanced Permeability and Retention (EPR) effect, using detailed investigations of the accumulation, distribution and retention of nanomedicines in solid tumors. Nanomedicine accumulation in preclinical tumors has been extensively studied; however, treatment efficacy will be heavily influenced by both the quantity of drug-loaded nanomedicines reaching the tumor as well as their spatial distribution throughout the tumor. It remains a challenge to image the heterogeneity of nanomedicine distribution in 3 dimensions within solid tumors with a high degree of spatial resolution using standard imaging approaches.
Methods: To achieve this, an ex vivo micro computed tomography (µCT) imaging approach was developed to visualize the intratumoral distribution of contrast agent-loaded PEGylated liposomes. Using this semi-quantitative method, whole 3-dimensional (3D) tumor liposome distribution was determined with 17 µm resolution in a phenotypically diverse panel of four preclinical xenograft and patient-derived explant (PDX) tumor models.
Results: High-resolution ex vivo μCT imaging revealed striking differences in liposome distribution within tumors in four models with different vascular patterns and densities, stromal contents, and microenvironment morphologies. Following intravenous dosing, the model with the highest density of pericyte-supported vessels showed the greatest liposome accumulation, while the model with vessels present in regions of high α-smooth muscle actin (αSMA) content presented with a large proportion of the liposomes at depths beyond the tumor periphery. The two models with an unsupported vascular network demonstrated a more restricted pattern of liposome distribution.
Conclusion: Taken together, vessel distribution and support (the latter indicative of functionality) appear to be key factors determining the accumulation and distribution pattern of liposomes in tumors. Our findings demonstrate that high-resolution 3D visualization of nanomedicine distribution is a useful tool for preclinical nanomedicine research, providing valuable insights into the influence of the tumor vasculature and microenvironment on nanomedicine localization.
Keywords: EPR, nanomedicine, distribution, µCT imaging, tumor microenvironment, vasculature.
 Global reach, higher impact
Global reach, higher impact