13.3
Impact Factor
Theranostics 2020; 10(2):829-840. doi:10.7150/thno.40195 This issue Cite
Research Paper
Histone deacetylase inhibition is synthetically lethal with arginine deprivation in pancreatic cancers with low argininosuccinate synthetase 1 expression
1. Department of Surgery, UCLA, Los Angeles, CA, 90095, USA.
2. Department of Pancreatic Surgery, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology (HUST), Hubei 430022, China
3. Ahmanson Translational Imaging Division, UCLA, Los Angeles, CA, 90095, USA.
4. Department of Molecular and Medical Pharmacology, UCLA, Los Angeles, CA, 90095, USA.
5. Department of Pancreatic and Thyroidal Surgery, Shengjing Hospital, China Medical University, Shenyang 110003, China
6. Department of Pathology and Laboratory Medicine, UCLA, Los Angeles, CA 90095, USA.
7. Jonsson Comprehensive Cancer Center, UCLA, Los Angeles, CA, 90095, USA
8. David Geffen School of Medicine, UCLA, Los Angeles, CA, 90095, USA.
Abstract
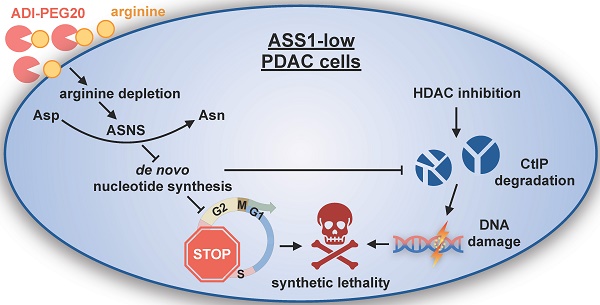
Arginine (Arg) deprivation is a promising therapeutic approach for tumors with low argininosuccinate synthetase 1 (ASS1) expression. However, its efficacy as a single agent therapy needs to be improved as resistance is frequently observed.
Methods: A tissue microarray was performed to assess ASS1 expression in surgical specimens of pancreatic ductal adenocarcinoma (PDAC) and its correlation with disease prognosis. An RNA-Seq analysis examined the role of ASS1 in regulating the global gene transcriptome. A high throughput screen of FDA-approved oncology drugs identified synthetic lethality between histone deacetylase (HDAC) inhibitors and Arg deprivation in PDAC cells with low ASS1 expression. We examined HDAC inhibitor panobinostat (PAN) and Arg deprivation in a panel of human PDAC cell lines, in ASS1-high and -knockdown/knockout isogenic models, in both anchorage-dependent and -independent cultures, and in multicellular complex cultures that model the PDAC tumor microenvironment. We examined the effects of combined Arg deprivation and PAN on DNA damage and the protein levels of key DNA repair enzymes. We also evaluated the efficacy of PAN and ADI-PEG20 (an Arg-degrading agent currently in Phase 2 clinical trials) in xenograft models with ASS1-low and -high PDAC tumors.
Results: Low ASS1 protein level is a negative prognostic indicator in PDAC. Arg deprivation in ASS1-deficient PDAC cells upregulated asparagine synthetase (ASNS) which redirected aspartate (Asp) from being used for de novo nucleotide biosynthesis, thus causing nucleotide insufficiency and impairing cell cycle S-phase progression. Comprehensively validated, HDAC inhibitors and Arg deprivation showed synthetic lethality in ASS1-low PDAC cells. Mechanistically, combined Arg deprivation and HDAC inhibition triggered degradation of a key DNA repair enzyme C-terminal-binding protein interacting protein (CtIP), resulting in DNA damage and apoptosis. In addition, S-phase-retained ASS1-low PDAC cells (due to Arg deprivation) were also sensitized to DNA damage, thus yielding effective cell death. Compared to single agents, the combination of PAN and ADI-PEG20 showed better efficacy in suppressing ASS1-low PDAC tumor growth in mouse xenograft models.
Conclusion: The combination of PAN and ADI-PEG20 is a rational translational therapeutic strategy for treating ASS1-low PDAC tumors through synergistic induction of DNA damage.
Keywords: HDAC inhibitor, arginine deprivation, pancreatic cancer, DNA damage, synthetic lethality
 Global reach, higher impact
Global reach, higher impact