13.3
Impact Factor
Theranostics 2020; 10(2):585-601. doi:10.7150/thno.36572 This issue Cite
Research Paper
In vivo liposomal delivery of PPARα/γ dual agonist tesaglitazar in a model of obesity enriches macrophage targeting and limits liver and kidney drug effects
1. Robert M. Berne Cardiovascular Research Center, University of Virginia, Charlottesville, Virginia, 22908, USA
2. Department of Pathology, University of Virginia, Charlottesville, VA, 22908, USA
3. Department of Biomedical Engineering, University of Virginia, Charlottesville, Virginia, 22908, USA
4. Research and Early Development, Cardiovascular, Renal and Metabolism (CVRM), BioPharmaceuticals R&D, AstraZeneca, Gothenburg, Sweden
5. Department of Medicine, Division of Cardiovascular Medicine, University of Virginia, Charlottesville, VA, USA
6. The Lundberg Laboratory for Diabetes Research, University of Gothenburg, Sweden
7. Wallenberg Centre for Molecular and Translational Medicine, University of Gothenburg, Sweden
Abstract
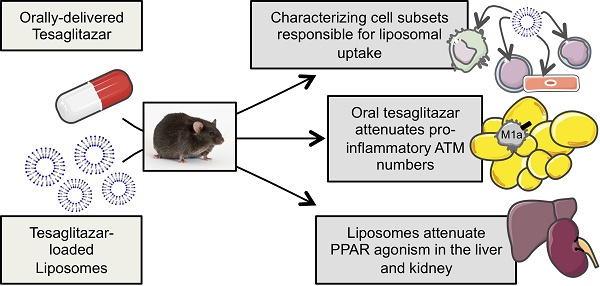
Macrophages are important regulators of obesity-associated inflammation and PPARα and -γ agonism in macrophages has anti-inflammatory effects. In this study, we tested the efficacy with which liposomal delivery could target the PPARα/γ dual agonist tesaglitazar to macrophages while reducing drug action in common sites of drug toxicity: the liver and kidney, and whether tesaglitazar had anti-inflammatory effects in an in vivo model of obesity-associated dysmetabolism.
Methods: Male leptin-deficient (ob/ob) mice were administered tesaglitazar or vehicle for one week in a standard oral formulation or encapsulated in liposomes. Following the end of treatment, circulating metabolic parameters were measured and pro-inflammatory adipose tissue macrophage populations were quantified by flow cytometry. Cellular uptake of liposomes in tissues was assessed using immunofluorescence and a broad panel of cell subset markers by flow cytometry. Finally, PPARα/γ gene target expression levels in the liver, kidney, and sorted macrophages were quantified to determine levels of drug targeting to and drug action in these tissues and cells.
Results: Administration of a standard oral formulation of tesaglitazar effectively treated symptoms of obesity-associated dysmetabolism and reduced the number of pro-inflammatory adipose tissue macrophages. Macrophages are the major cell type that took up liposomes with many other immune and stromal cell types taking up liposomes to a lesser extent. Liposome delivery of tesaglitazar did not have effects on inflammatory macrophages nor did it improve metabolic parameters to the extent of a standard oral formulation. Liposomal delivery did, however, attenuate effects on liver weight and liver and kidney expression of PPARα and -γ gene targets compared to oral delivery.
Conclusions: These findings reveal for the first time that tesaglitazar has anti-inflammatory effects on adipose tissue macrophage populations in vivo. These data also suggest that while nanoparticle delivery reduced off-target effects, yet the lack of tesaglitazar actions in non-targeted cells such (as hepatocytes and adipocytes) and the uptake of drug-loaded liposomes in many other cell types, albeit to a lesser extent, may have impacted overall therapeutic efficacy. This fulsome analysis of cellular uptake of tesaglitazar-loaded liposomes provides important lessons for future studies of liposome drug delivery.
Keywords: liposomes, tesaglitazar, peroxisome proliferator-activated receptors, obesity-associated dysmetabolism, macrophages
 Global reach, higher impact
Global reach, higher impact