13.3
Impact Factor
Theranostics 2020; 10(1):411-425. doi:10.7150/thno.33482 This issue Cite
Research Paper
Extracellular vesicle-mediated delivery of miR-101 inhibits lung metastasis in osteosarcoma
1. Department of Orthopedic Surgery, Orthopedic Oncology Institute of Chinese PLA, Tangdu Hospital, Air Force Medical University, Xi'an 710032, Shaanxi, China
2. Rehabilitation Center of Lintong Sanatorium of PLA, Xi'an 710600, Shaanxi, China
3. Department of Respiratory, Tangdu Hospital, Air Force Medical University, Xi'an 710032, Shaanxi, China
4. Department of Dermatology, the First Affiliated Hospital of Medical College of Xi'an Jiaotong University, Xi'an, 710061, Shaanxi, China
5. Department of Orthopedics, the 960th Hospital of the PLA Joint Logistics Support Force, Jinan 250000, Shandong, China
6. Department of Orthopedics, the Fourth Medical Center of Chinese PLA General Hospital, Beijing, 100048, China
*These authors contributed equally to this work.
Abstract
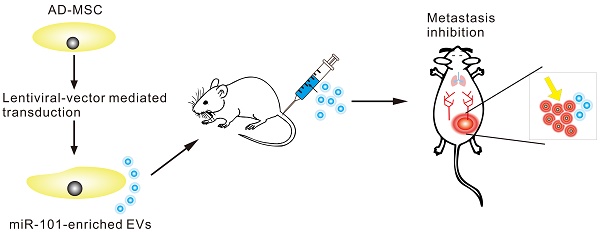
Rationale: Extracellular vesicles (EVs) have emerged as novel mediators of cell-to-cell communication that are capable of the stable transfer of therapeutic microRNAs (miRNAs), and thus, EVs hold immense promise as a miRNA delivery system for cancer therapy. Additionally, as miRNA-containing EVs are secreted into circulation, miRNAs contained within plasma EVs may represent ideal biomarkers for diseases. The objective of this study was to characterize a potential tumor suppressor miRNA, miR-101, and explore the potential of miR-101 delivery via EVs for in vivo therapy of metastatic osteosarcoma as well as the potential value of plasma EV-packaged miR-101 (EV-miR-101) level for predicting osteosarcoma metastasis.
Methods: The relationship of miR-101 expression and osteosarcoma progression was investigated in osteosarcoma specimens by in situ hybridization (ISH), and the potential inhibitory effect of miR-101 was further investigated using in vivo models. Using prediction software analysis, the mechanism of action of miR-101 in osteosarcoma was explored using quantitative reverse transcription polymerase chain reaction (qRT-PCR), western blotting and dual-luciferase assay. Adipose tissue-derived mesenchymal stromal cells (AD-MSCs) were transduced with lentiviral particles to obtain miR-101-enriched EVs. A Transwell assay and lung metastasis models of osteosarcoma were used to observe the effect of miR-101-enriched EVs on osteosarcoma invasiveness and metastasis. Detection of plasma EV-miR-101 levels was carried out in osteosarcoma patients and healthy controls by qRT-PCR.
Results: miR-101 expression was markedly lower in metastatic osteosarcoma specimens compared to non-metastatic specimens. Significantly fewer metastatic lung nodules were formed by Saos-2 cells overexpressing miR-101 and SOSP-9607 cells overexpressing miR-101 injected into mice. With increased miR-101 expression, B cell lymphoma 6 (BCL6) mRNA and protein expression levels were reduced, and miR-101 was found to exert its effects by directly targeting BCL6. AD-MSCs were successfully engineered to secrete miR-101-enriched EVs. Once taken up by osteosarcoma cells, these EVs showed suppressive effects on cell invasion and migration in vitro, and systemic administration of these EVs effectively suppressed metastasis in vivo with no significant side effects. Finally, the EV-miR-101 level was lower in osteosarcoma patients than in healthy controls and even lower in osteosarcoma patients with metastasis than in those without metastasis.
Conclusion: Our data support the function of miR-101 as a tumor suppressor in osteosarcoma via downregulation of BCL6. AD-MSC derived miR-101-enriched EVs represent a potential innovative therapy for metastatic osteosarcoma. EV-miR-101 also represents a promising circulating biomarker of osteosarcoma metastasis.
Keywords: extracellular vesicle, mesenchymal stromal cell, miR-101, osteosarcoma
 Global reach, higher impact
Global reach, higher impact