13.3
Impact Factor
Theranostics 2020; 10(1):62-73. doi:10.7150/thno.35735 This issue Cite
Research Paper
Ultra- small Pyropheophorbide- a Nanodots for Near- infrared Fluorescence/Photoacoustic Imaging-guided Photodynamic Therapy
1. School of Chemistry, Institute of Science and Center of Excellent-Advanced Functional Materials, Suranaree University of Technology, Nakhon Ratchasima 30000, Thailand.
2. Institute of Functional Nano & Soft Materials (FUNSOM), Collaborative Innovation Center of Suzhou Nano Science and Technology, Soochow University, Suzhou, Jiangsu 215123, China.
Abstract
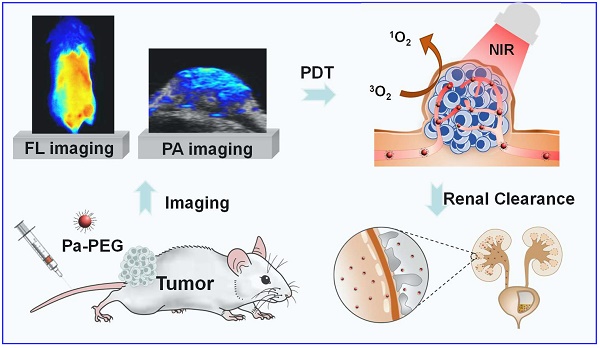
Rationale: Nanoparticles (NPs) that are rapidly eliminated from the body offer great potential in clinical test. Renal excretion of small particles is preferable over other clearance pathways to minimize potential toxicity. Thus, there is a significant demand to prepare ultra-small theranostic agents with renal clearance behaviors.
Method: In this work, we report a facile method to prepare NPs with ultra-small size that show renal clearable behavior for imaging-guided photodynamic therapy (PDT). Pyropheophorbide-a (Pa), a deep red photosensitizer was functionalized with polyethylene glycol (PEG) to obtain Pa-PEG. The prepared NPs formed ultra-small nanodots in aqueous solution and showed red-shifted absorbance that enabling efficient singlet oxygen generation upon light irradiation.
Results: In vitro studies revealed good photodynamic therapy (PDT) effect of these Pa-PEG nanodots. Most of the cancer cells incubated with Pa-PEG nanodots were destroyed after being exposed to the irradiated light. Utilizing the optical properties of such Pa-PEG nanodots, in vivo photoacoustic (PA) and fluorescence (FL) imaging techniques were used to assess the optimal time for PDT treatment after intravenous (i.v.) injection of the nanodots. As monitored by the PA/FL dual-modal imaging, the nanodots could accumulate at the tumor site and reach the maximum concentration at 8 h post injection. Finally, the tumors on mice treated with Pa-PEG nanodots were effectively inhibited by PDT treatment. Moreover, Pa-PEG nanodots showed high PA/FL signals in kidneys implying these ultra-small nanodots could be excreted out of the body via renal clearance.
Conclusion: We demonstrated the excellent properties of Pa-PEG nanodots that can be an in vivo imaging-guided PDT agent with renal clearable behavior for potential future clinical translation.
Keywords: Pa-PEG ultra-small nanoparticles, Dual-modal imaging, Photodynamic therapy, Renal clearance, toxicity
 Global reach, higher impact
Global reach, higher impact