13.3
Impact Factor
Theranostics 2020; 10(1):36-49. doi:10.7150/thno.37301 This issue Cite
Research Paper
Liver-targeted delivery of TSG-6 by calcium phosphate nanoparticles for the management of liver fibrosis
1. Department of Gastroenterology, Hepatology and Nutrition, Shanghai Children's Hospital, Shanghai Jiao Tong University, Shanghai 200062, China
2. Xijing Hospital of Digestive Diseases, State Key Laboratory of Cancer Biology, Fourth Military Medical University, Xi'an 710032, China
3. Marshall Laboratory of Biomedical Engineering, International Cancer Center, Laboratory of Evolutionary Theranostics (LET), School of Biomedical Engineering, Shenzhen University Health Science Center, Shenzhen, 518060, China
4. Endoscopy Center of Zhongshan Hospital, Fudan University, Shanghai 200032, China
*These authors contributed equally to this work.
Abstract
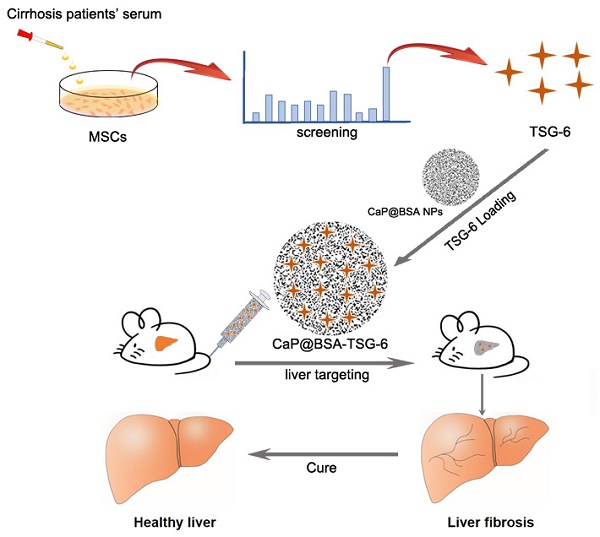
Mesenchymal stem cells (MSCs) transplantation is a promising antifibrotic strategy but facing clinical controversies. Inspired by advances in nanomedicine, we aimed to bypass these clinical barriers of MSCs by identifying the key antifibrotic molecule of MSCs and developing a specific liver-targeting nanocarrier.
Methods: Cytokines secreted by MSCs were examined with serum stimulation of cirrhotic patients. Immunohistochemistry, microarray, immunoblotting, and quantitative real-time PCR (qRT-PCR) were applied to identify the critical antifibrotic cytokine and to discover its role in modulating antifibrotic effects. Biomineralization method was used to prepare calcium phosphate nanoparticles (NPs). The targeting and therapeutic efficiency of NPs were evaluated by in vivo imaging and biochemical studies on fibrotic mice induced by CCl4.
Results: The stimulated MSCs exhibited high-level expression of Tumor necrosis factor (TNF)-stimulated gene 6 (TSG-6). On animal study, exogenous administration of TSG-6 alone can ameliorate liver fibrosis while TSG-6 knocked MSCs (Lv-TSG-6 MSCs) lost antifibrotic effects. Further studies verified the importance of TSG-6 and identified its antifibrotic mechanism by modulating M2 macrophages and increasing matrix metalloproteinase 12 (MMP12) expression. Additionally, we found a feedback loop between TSG-6, MMP12 and pro-inflammatory cytokines (TNF-α, IL-6, and IL-1β), which may improve our understanding of the aggravating process of cirrhosis and antifibrotic mechanisms of TSG-6 and MSCs. Based on these findings, we developed calcium phosphate nanoparticles (CaP@BSA NPs) by biomineralization method using bovine serum albumin (BSA) as the biotemplate. Imaging tracking and drug loading studies showed specific liver targeting and high TSG-6 loading efficacy of as-prepared CaP@BSA NPs. In vivo therapeutic study further demonstrated the improved therapeutic effects of TSG-6 loaded CaP@BSA.
Conclusions: TSG-6 was a major antifibrotic cytokine of MSCs, TSG-6 loaded CaP@BSA NPs showed specific liver accumulation and improved therapeutic effects, which indicated translational potentials of CaP@BSA as a promising drug carrier for the liver disease management.
Keywords: Liver fibrosis, Tumor necrosis factor-stimulated gene 6 (TSG-6), Calcium phosphate nanoparticle, Liver targeting delivery, matrix metalloproteinase 12 (MMP12), bovine serum albumin (BSA)
 Global reach, higher impact
Global reach, higher impact