13.3
Impact Factor
Theranostics 2019; 9(26):8426-8436. doi:10.7150/thno.35434 This issue Cite
Research Paper
Development of Adamantane-Conjugated TLR7/8 Agonists for Supramolecular Delivery and Cancer Immunotherapy
1. Center for Systems Biology, Massachusetts General Hospital, Boston, MA 02114, USA
2. Graduate Program in Immunology, Harvard Medical School, Boston, MA 02115, USA
3. Department of Radiology, Massachusetts General Hospital, Boston, MA 02114, USA
4. Department of Systems Biology, Harvard Medical School, Boston, MA 02115, USA
Abstract
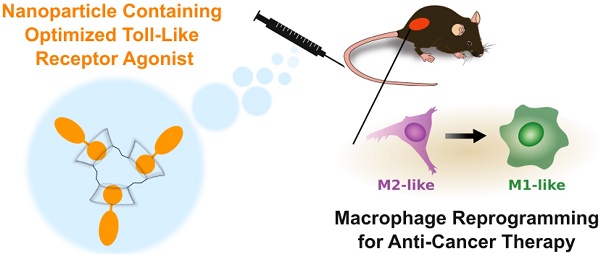
Tumor-associated macrophages (TAMs) are often abundant in solid cancers, assuming an immunosuppressive (M2-like) phenotype which supports tumor growth and immune escape. Recent methods have focused on identification of means (e.g., drugs, nanomaterials) that polarize TAMs to a tumor suppressive (M1-like) phenotype; however, reducing the systemic side effects of these therapies and enabling their delivery to TAMs has remained a challenge.
Methods: Here, we develop R848-Ad, an adamantane-modified derivative of the toll-like receptor (TLR) 7/8 agonist resiquimod (R848) through iterative drug screening against reporter cell lines. The adamantane undergoes guest-host interaction with cyclodextrin nanoparticles (CDNPs), enabling drug loading under aqueous conditions and TAM-targeted drug delivery. Therapeutic efficacy and systemic side effects were examined in a murine MC38 cancer model.
Results: R848-Ad retained macrophage polarizing activity through agonization of TLR7/8, and the adamantane moiety improved drug affinity for the CDNP. In preclinical studies, nanoformulated R848-Ad resulted in a drastic reduction in measurable systemic effects (loss of body weight) relative to similarly formulated R848 alone while arresting tumor growth.
Conclusions: The findings demonstrate the ability of strong nanoparticle-drug interactions to limit systemic toxicity of TLR agonists while simultaneously maintaining therapeutic efficacy.
Keywords: nanoparticle, cyclodextrin, drug screening, drug delivery, immunotherapy, macrophage
 Global reach, higher impact
Global reach, higher impact