13.3
Impact Factor
Theranostics 2019; 9(26):8344-8361. doi:10.7150/thno.30707 This issue Cite
Research Paper
p300/CBP inhibitor A-485 alleviates acute liver injury by regulating macrophage activation and polarization
1. Department of Gastroenterology, Xinhua Hospital, School of Medicine, Shanghai Jiaotong University, 1665 Kongjiang Road, Shanghai 200092, China
2. Drug Discovery and Design Center, State Key Laboratory of Drug Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, 555 Zuchongzhi Road, Shanghai 201203, China
3. University of Chinese Academy of Sciences, 19 Yuquan Road, Beijing 100049, China
4. School of Pharmacy, Fujian Medical University, Fuzhou, China.
5. School of Pharmacy, Guiyang University of Traditional Chinese Medicine, South Dong Qing Road, Huaxi District, Guizhou 550025, China.
6. Key Laboratory of Metabolism and Molecular Medicine, the Ministry of Education, Department of Biochemistry and Molecular Biology, School of Basic Medical Sciences, Huashan Hospital, Fudan University, Shanghai 200032, China
#These authors contributed equally to this work.
Abstract
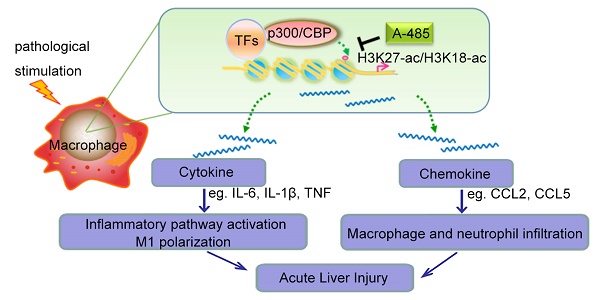
High morbidity and mortality are associated with acute liver injury (ALI) for which no effective targeted drugs or pharmacotherapies are available. Discovery of potential therapeutic targets as well as inhibitors that can alleviate ALI is imperative. As excessive inflammatory cytokines released by macrophages are a critical cause of liver injury, we aimed to find novel compounds that could inhibit macrophage expression of inflammatory cytokines and alleviate liver injury.
Methods: A high throughput assay was established to screen a small molecule inhibitor library of epigenetic targets. A highly selective catalytic p300/CBP inhibitor A-485 was identified as a potent hit in vitro and administrated to the lipopolysaccharide (LPS)/D-galactosamine (GalN)-induced mice in vivo. For in vitro analysis, RAW264.7 cells and primary BMDM cells exposed to LPS were co-incubated with A-485. A model of acute liver injury induced by LPS and GalN was used for evaluation of in vivo treatment efficacy.
Results: A-485 inhibited LPS-induced inflammatory cytokine expression in a concentration-dependent manner in vitro. Significantly, A-485 administration alleviated histopathological abnormalities, lowered plasma aminotransferases, and improved the survival rate in the LPS/GalN-stimulated mice. Integrative ChIP-Seq and transcriptome analysis in the ALI animal model and macrophages revealed that A-485 preferentially blocked transcriptional activation of a broad set of pathologic genes enriched in inflammation-related signaling networks. Significant inhibition of H3K27ac/H3K18ac at promoter regions of these pivotal inflammatory genes was observed, in line with their suppressed transcription after A-485 treatment. Reduced expression of these pathological pro-inflammatory genes resulted in a decrease in inflammatory pathway activation, M1 polarization as well as reduced leukocyte infiltration in ALI mouse model, which accounted for the protective effects of A-485 on liver injury.
Conclusion: Using a novel strategy targeting macrophage inflammatory activation and cytokine expression, we established a high-throughput screening assay to discover potential candidates for ALI treatment. We demonstrated that A-485, which targeted pathological inflammatory signaling networks at the level of chromatin, was pharmacologically effective in vivo and in vitro. Our study thus provided a novel target as well as a potential drug candidate for the treatment of liver injury and possibly for other acute inflammatory diseases.
Keywords: liver injury, inflammation, macrophages, epigenetics, acetyltransferase inhibitor
 Global reach, higher impact
Global reach, higher impact