13.3
Impact Factor
Theranostics 2019; 9(25):7906-7923. doi:10.7150/thno.38425 This issue Cite
Review
Recent advances and challenges of repurposing nanoparticle-based drug delivery systems to enhance cancer immunotherapy
1. Center for Theragnosis, Biomedical Research Institute, Korea Institute of Science and Technology (KIST), 5, Hwarangno 14-gil, Seongbuk-gu, Seoul, 02792, Republic of Korea
2. KU-KIST Graduate School of Converging Science and Technology, Korea University, 145, Anam-ro, Seongbuk-gu, Seoul, 02841, Republic of Korea.
*These authors contributed equally to this work
Received 2019-9-10; Accepted 2019-9-17; Published 2019-10-16
Abstract
Cancer immunotherapy is an attractive treatment option under clinical settings. However, the major challenges of immunotherapy include limited patient response, limited tumor specificity, immune-related adverse events, and immunosuppressive tumor microenvironment. Therefore, nanoparticle (NP)-based drug delivery has been used to not only increase the efficacy of immunotherapeutic agents, but it also significantly reduces the toxicity. In particular, NP-based drug delivery systems alter the pharmacokinetic (PK) profile of encapsulated or conjugated immunotherapeutic agents to targeted cancer cells or immune cells and facilitate the delivery of multiple therapeutic combinations to targeted cells using single NPs. Recently, advanced NP-based drug delivery systems were effectively utilized in cancer immunotherapy to reduce the toxic side effects and immune-related adverse events. Repurposing these NPs as delivery systems of immunotherapeutic agents may overcome the limitations of current cancer immunotherapy. In this review, we focus on recent advances in NP-based immunotherapeutic delivery systems, such as immunogenic cell death (ICD)-inducing drugs, cytokines and adjuvants for promising cancer immunotherapy. Finally, we discuss the challenges facing current NP-based drug delivery systems that need to be addressed for successful clinical application.
Keywords: Nanoparticles, drug delivery system, immunogenic cell death, adjuvants, cytokines, and cancer immunotherapy
1. Introduction
Cancer immunotherapy is emerging as an attractive treatment option for patients diagnosed with cancer, resulting in dramatic clinical results. The remarkable success of monoclonal antibodies as immune checkpoint inhibitors in clinical settings, and their subsequent approval by the United States Food and Drug Administration (FDA) has changed the landscape of cancer treatment [1, 2]. However, the benefit of most cancer immunotherapies has been limited to only a minority of patients and indications [3]. To overcome this poor efficacy of cancer immunotherapy, several attempts have been made using combination therapies consisting of multiple immune checkpoint inhibitors in clinical settings. Based on preclinical and clinical studies, combination therapy usually enhances therapeutic efficacy in cancer treatment, but can also lead to immune-related adverse effects in addition to the high cost involved. In addition, many immune checkpoint inhibitors administered systemically have limited tumor specificity, which can cause off-target adverse effects due to immune reactions against normal tissues [4-6]. The off-target adverse effects can range from relatively minor conditions such as skin redness or blisters, to severe adverse effects such as pneumonitis, colitis, and endocrinopathies [7, 8]. Therefore, novel approaches are urgently required to increase the efficacy of cancer immunotherapy by minimizing off-target adverse effects.
Various NP-based drug delivery systems have been extensively developed as vehicles for targeted cancer delivery of anticancer agents, such as chemical drugs, small interfering RNAs, DNAs, cytokines, and antibodies for over 30 years [9-12]. They have demonstrated tumor-specific delivery of anticancer agents, owing to the preferential accumulation of NPs at targeted tumors and enhanced permeability and retention (EPR) effect [13]. In addition to EPR effect, NPs have been used to actively target drug delivery to the desired cancer cells via specific binding to target receptors overexpressed on the surface of cancer cells. Active targeting may be achieved by simply introducing targeting moieties (e.g., peptides, aptamers, antibodies) on the surface of NPs [14]. This target-specific delivery of anticancer drugs based on EPR and active targeting has been shown to greatly enhance therapeutic efficacy, and successfully diminish undesirable off-target toxicity [15-17].
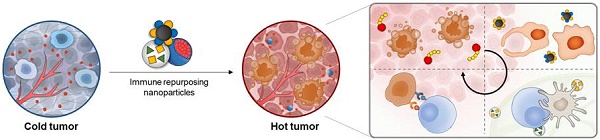
Repurposing these NP-based drug delivery systems for immunotherapeutic agents to target the immune system may overcome the lower efficacy, immune-related adverse events, and off-targeted toxicity of currently used agents (Figure 1). Notably, the use of NPs for immunotherapeutic delivery can offer the following benefits. First, NPs containing one or more immunotherapeutic agents can easily target the desired cancer or immune cells. The targeted delivery of NPs to cancer cells can be accomplished based on EPR effect and active targeting strategies such as tumor-targeting nanomedicine. Targeted delivery of NPs to immune cells or immune cell subsets can be further enhanced via chemical or physical modification of immune system-targeting ligands that specifically bind to overexpressed receptors on the surface of target cancer or immune cells [18-20]. Several studies have shown that fine-tuning of sizes, shapes, surface charges, and hydrophobicity of NPs successfully improved the delivery of immunotherapeutic agents into tumor tissues or lymph nodes (LNs) [21, 22]. Second, each NP can be designed to incorporate multiple immunotherapeutic agents for delivery to cancer targets cancer or immune cells simultaneously [23]. Many studies have demonstrated that co-delivery of immunotherapeutic agents within a single cell enhanced the immune response and optimized the antigen processing and presentation. Finally, NPs containing immunotherapeutic agents show controlled drug release in response to complex tumor microenvironments (TME) (e.g., enzymes, hypoxia and acidic pH) or external stimuli (e.g., light, ultrasound, and electricity), which can greatly increase the efficiency of targeted drug delivery in cancer immunotherapy [24]. Therefore, NP-based drug delivery systems can be used to enhance the efficacy of immunotherapeutic agents such as antibodies and cytokines in cancer immunotherapy.
In this review, we tried to discuss repurposing inherent properties (e.g. encapsulation of chemodrugs, target-specific delivery, controlling PK of drugs, etc.) of NP-based delivery systems to immunotherapeutics for improving therapeutic efficacy in cancer immunotherapy. In this point of view, recent studies which provided implications for overcoming current challenges in immunotherapeutics were selected and categorized into three types (Immunogenic cell death-inducing cytotoxic NPs, cytokines and cytokine-like immune modulator NPs, and adjuvants). Furthermore, we highlighted the potential of NP-based delivery systems that could overcome the current limitations of cancer immunotherapy. Finally, we discussed the expected challenges associated with repurposing such drug delivery systems for cancer immunotherapy, including low levels of tumor targeting, complex mechanism of therapeutic efficiency and limited commercial success.
2. Advanced NP-based drug delivery systems of immunogenic cell death (ICD)-inducing cytotoxic drugs
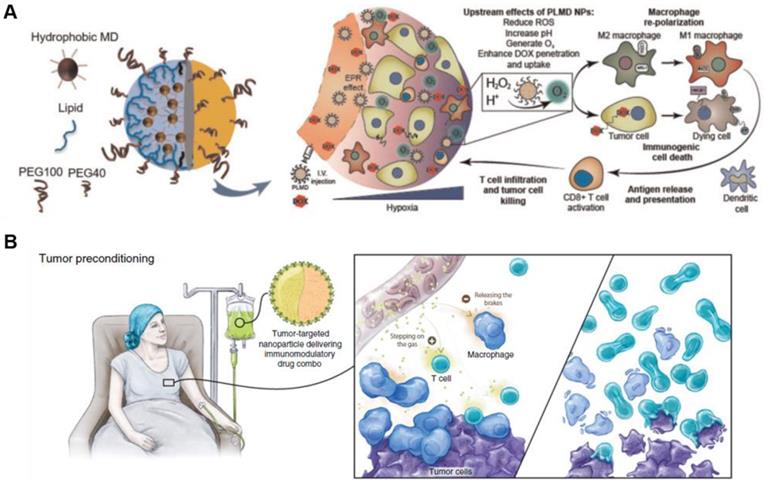
Conventional immunotherapeutic agents including immune checkpoint inhibitors fail to completely eliminate most types of tumors in the body, and show limited therapeutic efficacy [25, 26]. Clinical studies have shown that only a fraction (10-38%) of cancer patients respond to current treatment using immune checkpoint inhibitors [27-31]. Additional synergistic mechanisms are needed to improve the therapeutic efficacy of current immunotherapy of cancer patients co-administered with pre-existing immune checkpoint inhibitors or ICD-inducing cytotoxic drugs [27]. Cytotoxic drugs such as doxorubicin, 5-fluorouracil, gemcitabine, paclitaxel, mitoxantrone and oxaliplatin elicit an immune response by activating apoptotic pathways and triggering ICD [32, 33]. ICD in tumors induced with cytotoxic drugs triggers an antitumor immune response, leading to activation of the immune system against tumors [34, 35]. The ICD in the tumor is characterized by damage-associated molecular patterns (DAMPs), including overexpressed high mobility group protein B1 (HMGB1), cell surface-exposed calreticulin (CRT) and adenosine triphosphate (ATP) [36, 37]. In particular, surface‐exposed CRT and secreted ATP regulate immunogenicity in chemotherapy-induced apoptosis. Also, crucial DAMPs such as extracellular HMGB1 contribute to enhanced ICD by binding to toll-like receptor 4 (TLR4) on dendritic cells, potentiating cancer immunotherapy. In general, anticancer chemotherapy has a lethal effect on malignant cancer cells as well as other normal cells, leading to high off-target toxicity in normal and immune cells. Therefore, simple combination therapies comprising pre-existing cytotoxic drugs lead to severe clinical challenges such as unwanted systemic toxicity and immune suppression. In particular, repurposing NP-based drug delivery systems of ICD-inducing anticancer drugs facilitates cancer immunotherapy for maximal therapeutic efficacy with low toxicity. NP-based delivery of cytotoxic drugs showed enhanced drug efficacy and diminished unwanted off-target toxicity, due to their tumor targeting ability in many preclinical and clinical tests (Figure 2) [15, 38]. The NP-based tumor-targeting delivery systems increased the retention time of cytotoxic agents at the tumor site and the rapid and enhanced cellular uptake of NPs boosted the tumor-specific immune response. It is well known that macromolecules including NPs and nanocomplexes enter the tumor interstitial space and accumulate in the tumor tissue, contributing to the EPR effect [39-41]. Tumor-specific delivery of cytotoxic agents alter the immunosuppressive conditions in tumor tissues by facilitating tumor immunogenicity of antigens in many cancers and enhancing ICD to potentiate cancer immunotherapy [42]. For example, polymer-lipid doxorubicin-encapsulated manganese dioxide NPs were successfully delivered to the tumor tissue due to the EPR effect, reversing immunosuppressive conditions in an orthotopic mouse model of breast tumor (Figure 3A) [43]. In this study, attenuation of hypoxia and acidosis via NP treatment led to remodeling of the TME and boosted T-cell activity, inhibiting tumor progression. This type of tumor-specific delivery and immunogenic cytotoxic effect of NP-based delivery system may facilitate cancer immunotherapies or other T cell therapies used to treat tumors (Figure 3B) [44]. Given the merits of tumor-specific EPR effect of NPs, ICD-inducing delivery platform can be explored to maximize the clinical outcomes of various immunotherapies. In addition to killing cancer cells, NP-based drug delivery systems for ICD-inducing cytotoxic drug can modulate immune reaction in TMEs, resulting in anti-tumor immune response [45]. Several new developments have been reported in NP-based drug delivery systems to carry cytotoxic drugs to targeted cancer cells [46-49]. This section focuses on recent advances in NP-based delivery systems of cytotoxic drugs leading to enhanced ICD in TME.
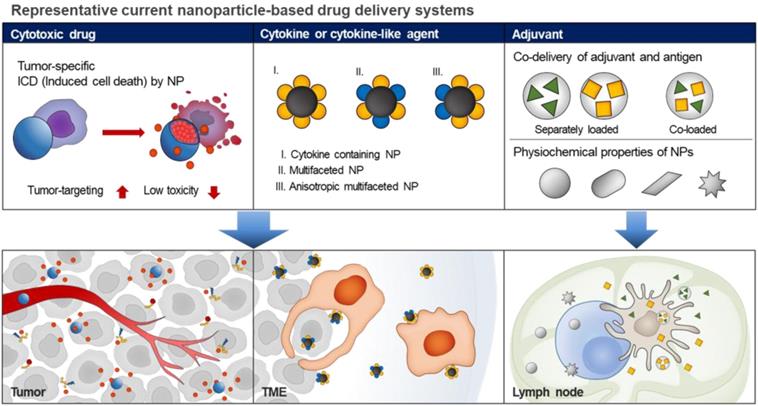
Schematic illustrations of current nanoparticle-based drug delivery systems for cancer immunotherapy. Based on the EPR effect and active targeting strategies, the current nanoparticle-based drug delivery systems can be used for targeted delivery of cytotoxic drugs to the tumor tissue, resulting in ICD induction. In addition, the nanoparticle-based cytokine delivery system for the delivery of multiple immunotherapeutic agents to target tumor cells or immune cells, results in immune responses. In the case of adjuvant delivery, the nanoparticle-based delivery systems with fine-tuned sizes, shapes, surface charges, and hydrophobicity can be used for the delivery of immunotherapeutics into tumor tissues or lymph nodes.
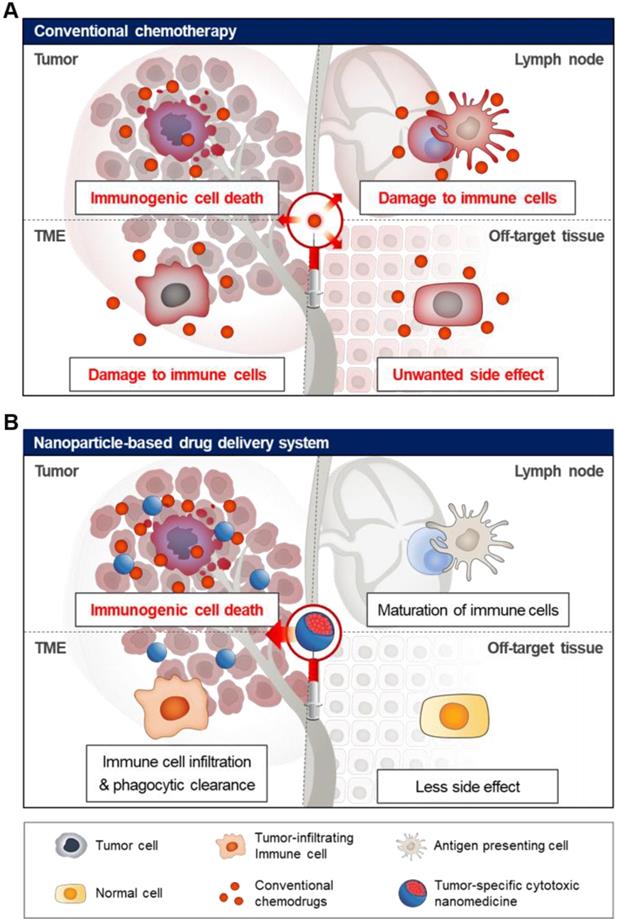
Schematic illustration of off-target toxicity in conventional chemotherapy. (A) Chemotherapy generally acts by killing fast-growing cells including malignant tumor cells and normal cells, leading to high off-target toxicity. (B) Nanoparticle-based drug delivery system can be used for tumor-specific delivery of chemotherapeutic drugs using the EPR effect and active targeting strategy, resulting in a significant increase in drug levels in the tumor tissue. Finally, cytotoxic chemotherapy induces tumor-specific immunogenic cell death in the tumor tissue, activating immune response with fewer side effects.
Recent studies show that novel NP-based delivery systems can be combined with immune modulators to enhance ICD response and antitumor immunity [50]. For example, Zhang's group recently showed that nano-sized prodrugs consisting of doxorubicin, matrix metalloproteinase (MMP)-cleavable peptide and hyaluronic acid (HA) initiated antitumor immune response via upregulation of interferon-γ (IFN-γ) and programmed cell death protein-ligand 1 (PD-L1) [51]. The prodrug-based NPs with a uniform size successfully induced the release of ICD-associated molecules, including HMGB1, IFN, and tumor necrosis factor (TNF) α in TME resulting in enhanced immune response by activated T cells. When combined with anti-PD-1 antibody (a-PD1), prodrug-based NPs elicited strong antitumor immune response and antitumor immunity, mediated by robust tumor-infiltrating lymphocytes (TILs). Thus, NP-based delivery systems carrying cytotoxic drugs can be used to facilitate ICD via mature antigen-presenting cells (APCs) in TMEs, However, the TME shows distinct mechanisms of immunosuppression, and infiltration by immunosuppressive cells, such as myeloid-derived suppressive cells, tumor-associated macrophages, and regulatory T cells [52]. Repurposing the NP-based drug delivery systems of cytotoxic agents targeting the immune system may overcome the lower efficacy and potential toxicity of current cancer immunotherapeutics. To boost the ICD response and increase the antitumor immunity, immunosuppressive pathway inhibitors may also be used together with chemotherapeutic drugs. Nel et al. recently utilized mesoporous silica NPs (MSNPs) with oxaliplatin (OX) and immunosuppressive indoleamine 2,3-dioxygenase (IDO) pathway inhibitor (indoximod; IND) to treat orthotopic pancreatic ductal adenocarcinoma [53]. In the study, the MSNPs induced ICD successfully and recruited several cytotoxic T lymphocytes in TME, resulting in induction of antitumor immunity. The subsequent release of DAMPs including HMGB-1 and ATP from tumor cells by the action of NPs provided immunogenic stimuli to the antigen presenting DC. Also, CRT expression on the dying cell surfaces in TME promoted an “eat-me” signal for the cellular uptake of DCs, which resulted in significant enhancement of the ICD effect by the IND. This study indicated that the combination therapy using NP-based delivery system containing both OX and IND resulted in a synergetic immunotherapy response by enabling induction of ICD along with a reversal of immune-suppressive effects. In addition, MSNPs improved the pharmacokinetics (PK) and tissue distribution of both OX and IND, which may increase the antitumor immunity synergistically.
Modulation of functional nanoparticles with anti-cancer agents enhances therapeutic efficacy and boosts antitumor immunity. (A) Treatment using PLMD combined with doxorubicin leads to accumulation in the tumor tissue via EPR effect, reducing hypoxia and acidosis. More importantly, it enhances doxorubicin-induced immunogenic cell death-promoting macrophage phenotype polarization from M1 to M2 type. Adapted with permission from [43], Copyright 2019 Oxford University Press. (B) The schematic illustration shows that targeted nanoparticles potentially improve immunotherapy by remodeling TME and stimulating key antitumor effector cells. Adapted with permission from [44], Copyright 2018 AACR publications.
Various NP-based drug delivery systems containing chemotherapeutic agents have successfully modulated the immune response against tumors via EPR effect or active targeting mechanism, compared with free chemotherapeutic agents. These NPs containing polymers, inorganic, and bio-derived nanomaterials effectively deliver immune-modulating agents into tumors [54]. In particular, exosomes are the representative endogenous NPs that play a great role in the delivery of anticancer drugs combined with immune modulators because they contain various immune-modulating cytokines derived from cells [54, 55]. For example, exosomes isolated from M1- macrophages directly enhanced the antitumor efficacy of chemotherapeutics in TME [56]. In this case, the antitumor efficacy of paclitaxel (PTX) was significantly improved in tumor-bearing mice following simultaneous delivery of ICD-inducing PTX and pro-inflammatory cytokines to the targeted tumor site by the exosomes. The TME-activated binary cooperative NP with acidity-induced cleavage of poly(ethylene glycol) shell and glutathione-mediated linker is another new NP-based delivery system used to modulate immunosuppressive TME. The enhanced accumulation of self-assembled cytotoxic prodrugs in TME triggered ICD of targeted cancer cells [57]. In the study, the tumor-specific action of dual‐activatable prodrug NPs elicited antitumor immunity resulting in enhanced immunotherapy, and intratumoral accumulation of cytotoxic T lymphocytes. The TME-specific nano-prodrugs exhibit great potential for clinical application by effectively delivering TME-activated prodrug to targeted tumor cells, resulted in enhanced immunogenicity of tumor cells without affecting normal immune cells.
Lastly, several studies showed that encapsulation of a cytotoxic agent in NPs eliminated undesirable immune side effects of peptides and liposomes. For example, drug delivery using encapsulated cytotoxic agent (doxorubicin) completely eliminated the lethal immunotoxicity of peptides and liposomes in ICR (Institute of cancer research) mice [58]. Lu et al reported that cyclic RGD peptide-based modification of liposomes induced acute systemic anaphylaxis, IgG immune complex-triggered complement activation, and cytokine release. Minimizing the ratio of cyclic RGD peptide, or decreasing the injection doses failed to resolve the acute systemic toxicity. However, encapsulated NPs of doxorubicin eliminated the systemic immune response by inhibiting immunotoxicity and antibody overproduction. Therefore, unwanted immunotoxicity of NPs can be controlled by encapsulation of cytotoxic drugs. Recent advances in NP-based delivery systems, therefore, increase therapeutic efficacy by triggering ICD in TME, and decreasing systemic toxicity by TME-specific delivery [15].
Recent advances demonstrated a variety of applications in cancer immunotherapy via targeted delivery of ICD-inducing agents with adjuvants. Furthermore, by delivering various immunotherapeutic agents with synergistic effect, they contribute to an immunogenic TME, resulting in antitumor immunity. However, the poor drug delivery efficiency and systemic toxicity of NP-based systems in cancer immunotherapy are still persistent challenges limiting their clinical application. Therefore, the tumor-specific design of NPs, which can improve the delivery of cytotoxic agents is crucial for successful cancer immunotherapy and development of immunostimulating agents for treatment of cancer patients.
3. Advanced NP-based drug delivery systems of cytokines and cytokine-like immune modulators
Cytokines are a broad category of small biomolecules that are important in cell signaling. In cancer therapy, among cytokines, IFNs, interleukins, and chemokines have been widely used as immunomodulating agents. Specifically, IFN-α, a representative FDA-approved cytokine has been used in the clinic to treat leukemia since 1986, and subsequently, recombinant interleukin-2 (IL-2) has been developed for cancer immunotherapy since 1992 [59-61]. However, these cytokines have short half-lives and limited stability, which can be overcome by using NP-based delivery systems [62, 63]. NP-based cytokine delivery can overcome limitations of conventional cytokine-based therapy such as short half-life, autoimmune attack and inflammatory immune reaction [64-67]. In addition, several NPs have shown potential to modulate and fine-tune inflammatory responses to facilitate their use as immune-modulating agents in anti-cancer treatment. Various studies have shown that cytokines are easily encapsulated or chemically conjugated to different NPs, preventing degradation of cytokines by enzymes in vivo . Conjugation of cytokines on the surface of NPs enables cytokine delivery to the target receptors on the cell surface. Moreover, NPs selectively deliver cytokines to target tissues via controlled release mechanisms [66, 68, 69]. For example, conjugation of IL-2 as T cell growth factor on the surface of hydroxyethyl starch nanocapsules (HES-D-IL-2) via copper-free click chemistry increased the binding affinity of IL-2 receptor compared with free IL-2, and enhanced the targeting efficiency of T cell populations [68]. In addition, T cell immune response is closely dependent on the number of conjugated IL-2 molecules on the nanocapsule surface. HES-D-IL-2 nanocapsules are significantly absorbed by activated CD4+ CD25+ T cells compared with naïve CD4+ CD25- T cells, resulting in enhancing activated T cell proliferation. This study indicates that the immune response of activated T cells can be modulated by controlling a number of ligands on the nanocapsule surface. Similarly, conjugation of tumor necrosis factor related apoptosis-inducing ligand (TRAIL) with the lipid nanocarrier membrane enhanced the potency of cytokines in cancer therapy [69]. The expression of TRAIL on the surface of the lipid nanocarriers effectively enhanced the pro-apoptotic activity of extrinsic TRAIL pathway and increased the activity of apoptosis-inducing caspases. Immunotherapeutic approaches using NP-based delivery system usually improved the targeting efficiency and PK/pharmacodynamics (PD) of encapsulated or conjugated cytokines in vivo [67, 70-73]. In order to maximize the therapeutic efficacy of cytokines, some NPs with cytokine-like ability have been developed in cancer immunotherapy. In this regard, recently developed NPs have been surface-modified using two or more surface moieties to investigate their role as cytokines. In particular, the development of a NP with two distinct faces (Janus-faced) and functions, represents a new direction for cancer immunotherapy [74]. The control of immune activity using multifaceted NPs suggests an immunomodulatory role. Therefore, in this section, we described recent advances in cytokine-like immune-modulating NPs as novel NP-based delivery systems in cancer immunotherapy.
Multifaceted NP platforms improve the selective delivery and targeting ability of many cytokine-like immune-modulating agents to target cancer and immune cells with efficacy. A variety of studies report the development of NPs by engineering cytokines via chemical conjugation or physical loading of various immune modulators such as antibodies, immune-related proteins and genes [67, 75, 76]. Multifaceted NPs containing two or more immune-modulating agents, which selectively target tumor tissues and inhibit tumor growth, have the potential to enhance antitumor immunity. Furthermore, the NPs are involved in immunotherapy as agonists or antagonists that specifically modulate the complex cancer immunity. Recently, multifaceted NPs have facilitated simultaneous targeting of lymphocytes and cancer cells. The Schneck's group developed NPs, stimulating T cells to kill tumor cells, by coating a single NP with tumor cell-binding antibody (a-human CD19) and loaded antigen-specific T cell-binding peptide (MHC-binding peptide) [75]. The multifaceted NPs were selectively targeted to tumor cells to stimulate antigen-specific T cells, which resulted in tumor regression. Moreover, multifaceted NPs readily control the complex binding affinities to different targeted cells by altering the ratios of various tumor antigens on the NP surface and boost the lethal effect of T cells against tumors. Kim et al. reported tumor eradication using multifaceted NPs by simultaneous targeting cancer cell-specific receptors and signaling phagocytosis of macrophages [77]. The multifaceted NP specifically targeted cancer cells overexpressing human epidermal growth factor receptor-2 and strongly stimulated professional APCs by calreticulin, which is a protein inducing phagocytosis. Consequently, phagocytosis by APCs was triggered by multifaced NPs, which are selectively bound to cancer cells, resulting in innate and adaptive immunity.
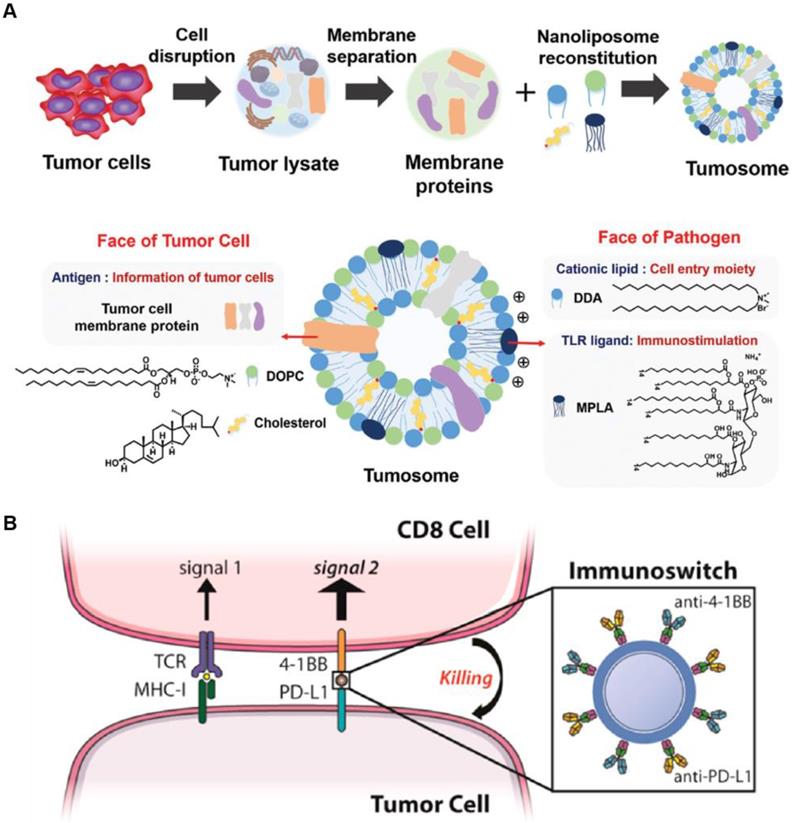
As an interesting multifaceted NP platform, cell-derived nanovesicles represent intracellular immune-modulating NPs that potentially enhance antitumor immunity [78, 79]. For example, multifaceted nanovesicles derived from macrophages or tumor cells were synthesized to boost endogenous immune response. M1 macrophage-derived nanovesicles effectively encapsulated M1 macrophage markers and mRNAs of pro-inflammatory factors, and replicated the function of M1 macrophages during immunotherapy [78]. The M1 macrophage-derived nanovesicles efficiently polarized M2 tumor-associated macrophages to antitumor M1 type macrophage and not only enhanced the secretion of antitumor cytokines but also significant suppressed the tumor growth. Alternatively, tumor cells derived from multifaceted nanovesicles have the potential to deliver tumor antigens and adjuvants to LNs resulting in an enhanced antitumor immune response [79]. The tumor cell-derived nanovesicle was composed of various proteins in the tumor cell membrane acting as tumor antigens, and a pathogen ligand, which facilitated antigen recognition by APCs (Figure 4A). In this study, multifaceted nanocarriers showed that incorporating the NPs with various immunomodulating agents, such as cancer cell-specific biomarkers, triggers an adaptive immune response.
The immunosuppressive TME hampers immune checkpoint inhibitor therapy and promotes metastasis of cancer cells [76, 78, 80]. The multifaceted NP platform can be used to overcome these limitations by modulating TME or targeting metastatic cells in the circulation. Schneck's group developed dual-targeting NPs, which simultaneously stimulate immune cells and block immune checkpoints on cancer cells. The multifaceted NPs were coated with two types of antibodies (anti-4-1BB and anti-PD-L1). The anti-4-1BB induces tumor-targeted CD8+ T cells to increase the secretion of cytokines including IFN-γ. The anti-PD-L1 significantly blocks the immunosuppressive pathway of cancer cells to prevent immune evasion from cytotoxic T cells (Figure 4B) [76]. Combination therapy with both antibodies using the NP platform significantly enhanced the therapeutic efficacy compared with conventional treatment using soluble anti-PD-L1 and anti-4-1BB. This study showed that multifaceted NPs reduce immunosuppression in the TME and stimulate the activity of T cells for effective cancer immunotherapy. Recently, King et al. developed multifaceted NPs coated with TRAIL and E-selectin adhesion molecules to target metastatic cancer cells [81]. E-selectin on NP surface specifically binds to leukocyte membrane, and therefore, TRAIL is circulated in the blood by attached to white blood cells and can evade renal clearance. By tethering NPs to leukocytes, this approach mimics the cytotoxic activity of tumor-targeted T cells, and effectively eradicates metastatic cancer cells from the blood. Taken together, the multifaceted NP platform, which has a great potential for specific targeting, reduces off-target effects while enhancing the efficacy of antitumor activity. Furthermore, multifaceted NPs can deliver various immune modulators, which act as cytokines in cancer immunotherapy.
Schematic illustration of multifaceted immune-modulating nanoparticles and immunoswitch nanoparticles. (A) Multifaceted nanocarriers derived from tumor cells are composed of tumor cell membrane proteins including tumor antigens and pathogen ligand, which act as immunostimulatory adjuvants. Adapted with permission from [79] copyright 2017 Wiley-VCH. (B) Multifaced nanoparticles act as immunoswitches coated with two types antibodies (anti-4-1BB and anti-PD-L1). The anti-4-1BB efficiently induces a T cell response and anti-PD-L1 significantly blocks the immunosuppressive pathway of tumor cells. Adapted with permission from [76] copyright 2017 American Chemical Society.
Utilization of Janus nanoparticles for cancer immunotherapy. (A) Janus nanoparticles were functionalized with alkyne-tagged anti-CD28 antibodies via azide groups via click chemistry. Super-resolution fluorescence images of the Janus nanoparticle show spatial segregation of anti-CD3 (red) and anti-CD28 (green) clusters in the nanoparticle. The spatial arrangement of ligands in the Janus nanoparticles facilitates simultaneous binding events including T cell activation. Adapted with permission from [83], Copyright 2017 The Royal Society of Chemistry. (B) SEM and fluorescence images of the Janus nanoparticles show the orientation on hydrophilic and hydrophobic substrates. The 100 nm Janus nanoparticles successfully induced defects in zwitterionic lipid bilayers. Adapted with permission from [84], Copyright 2018 ACS publications.
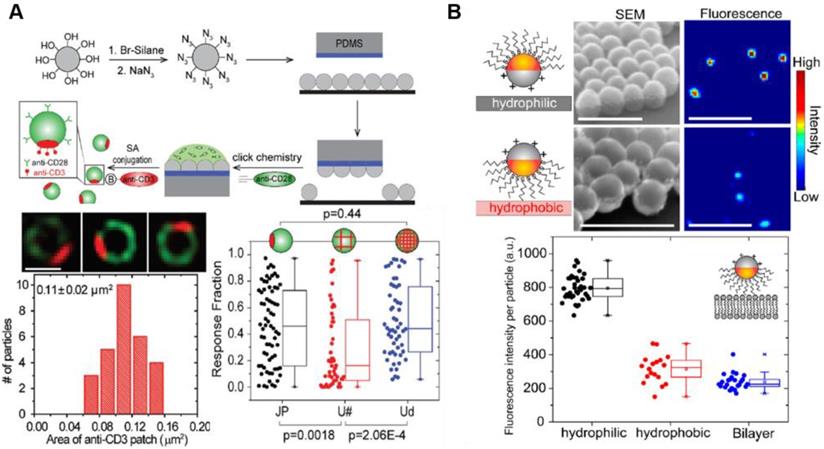
Recent studies have shown that a single NP with different surface characteristics exhibits a range of cytokine-like activities in cancer therapy [82]. Among multifaceted NP-based delivery systems, Janus NPs carrying surfaces with two or more distinct biochemical properties have been used to modulate NP-cell interactions similar to natural cytokines. Recent advances in cancer immunotherapy suggest that Janus NPs have a great potential in immune cell response. For example, Janus NPs with an increased surface density of ligands activated T cells significantly compared with uniformly coated NPs carrying the same number of ligands [83]. The spatial arrangement of ligands in the Janus NPs may affect simultaneous binding events including T cell activation for cancer immunotherapy, providing a new strategy to increase co-stimulatory immune response and T cell stimulation (Figure 5A) [83]. Until now, this approach was not used to design immune-modulating drugs due to the technical challenges associated with the fabrication of biomolecule clusters on NP surfaces. Recent advances in nanotechnology have enabled the design and synthesis of specialized functional NPs facilitating interaction with various immune cells. For example, Yu et al. showed that amphiphilic Janus NPs with different charges or hydrophobicity disrupted lipid bilayers more effectively than other uniformly coated NPs (Figure 5B) [84]. The Janus NPs measuring 100 nm in size induced defects in zwitterionic lipid bilayers at picomolar concentrations. Owing to their extraordinary heterostructure and surface modulation, they have been used in drug delivery associated with cancer immunotherapy [85, 86]. Designing NPs with distinct structural features and unique functionalities may provide a new approach in immunotherapy.
Recent studies showed that NPs effectively deliver cytokines and cytokine-like immune modulators to target cells, resulting in improving therapeutic efficacy as well as prolonged half-life and stability. However, the rational design of NPs requires reducing off-target effects and cytokine resistance for clinical application.
4. Advanced NP-based drug delivery systems of adjuvants
In cancer immunotherapy, adjuvants play a significant role in activating the APCs and generating a strong immune response [87, 88]. Thus, adjuvants have been widely used to formulate vaccines to enhance the immunogenicity of antigens and vaccine efficacy. However, adjuvants increase the risk of unwanted severe toxicity. Indeed, very few adjuvants have been approved for human use because of severe toxicity. Repurposing the NP-based delivery systems of adjuvants has been shown to significantly contribute to enhanced immunogenicity and diminished toxicity. In addition, NP-based adjuvant delivery with antigen can be used to channel the immune response either toward T helper cell type 1 (Th1) or T helper cell type 2 (Th2) [89].
Co-delivery of adjuvant and antigen is an effective therapeutic strategy in adjuvant-based immunotherapy [90-92]. Particle-based vaccines can be used to co-deliver adjuvant and antigen to the same APCs for selective uptake the antigen, using a relatively low amount of adjuvant for efficient immune response and reduce the risk of unwanted toxicity [93]. Furthermore, co-delivery of adjuvant and antigen using particle-based vaccine has been shown to promote antigen cross-presentation and cytotoxic CD8+ T cell activation [87]. The commonly used adjuvants in NPs for cancer immunotherapy include aluminum salts (alum), lipopolysaccharide (LPS), CpG oligodeoxynucleotides (CpG ODNs), layered double hydroxide (LDH), and polyinosinic:polycytidylic acid (poly I:C) [94, 95].
Schematic illustration of nanoparticle-based delivery strategies of adjuvant and antigen for efficient immune stimulation. (A) Albumin/AlbiVax nanocomplexes composed of AlbiCpG and AlbiAg bind to endogenous albumin in vivo . Albumin/AlbiVax nanocomplexes efficiently activated APCs inducing antitumor immunity by stimulating CD8+ T cells. Adapted with permission from [97] Copyright 2017, Springer Nature. (B) Synthetic sHDL nanodisk-based adjuvant and antigen co-delivery system. The sHDL nanodisks are composed of adjuvant CpG and tumor-specific neoantigens, which can be delivered to LNs. The sHDL nanodisks were absorbed by DCs followed by antigen cross-presentation. Finally, cytotoxic T cells were significantly activated to kill tumor cells. Adapted with permission from [98] Copyright 2017, Springer Nature.
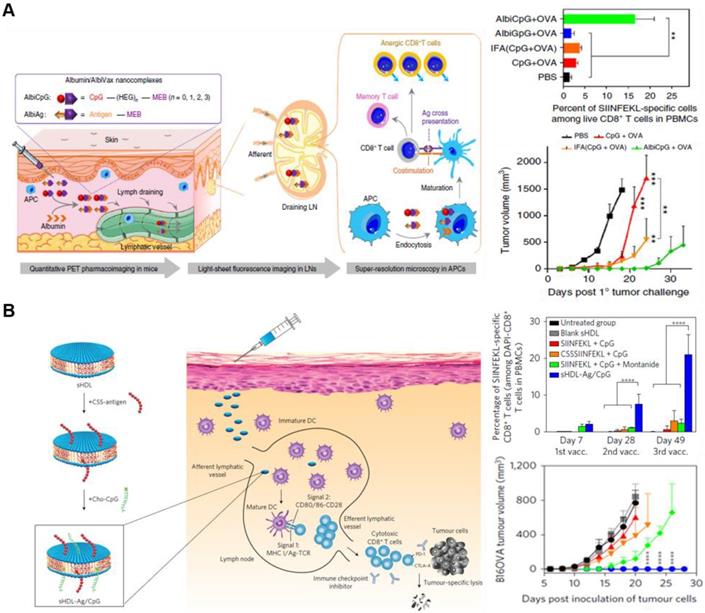
The adjuvant and antigen can be co-delivered in separate NPs or in a single NP. For example, Lim's group developed interesting synthetic vaccine NPs (SVNPs) that target LNs and enhance antitumor immunity [96]. SVNPs are composed of two types of NPs: one carrying the adjuvant, a toll-like receptor 3 (TLR3) agonist (poly I:C), and the other carrying the model tumor antigen, ovalbumin (OVA) prepared by electrostatic adsorption. Poly I:C is a double-stranded RNA and interacts with TLR3, which are expressed on leukocyte membranes. SVNPs showed higher cellular uptake into APCs, enhancing the secretion of type Ⅰ IFN-α and -β and pro-inflammatory cytokines compared with soluble poly I:C or OVA. Furthermore, Chen's group recently developed a noncomplex platform for efficient co-delivery of adjuvant and antigen using endogenous albumin. The albumin-binding vaccine (AlbiVax), consisting of albumin-binding adjuvant (AlbiCpG) and antigen (AlbiAg), is self-assembled with endogenous albumin in vivo (Figure 6A) [97]. The AlbiVax-based nanocomplexes were efficiently delivered to LNs and induced antigen-specific T cell responses in mice. Immunotherapy using albumin/AlbiVax nanocomplexes significantly enhanced the therapeutic effect. The adjuvant and antigen can also be co-delivered by loading them in the same nanocarrier. Moon et al. have shown that using synthetic high-density lipoprotein (sHDL) as a nanocarrier combined with adjuvant and neoantigen significantly improved the adjuvant delivery to LNs and antigen presentation on APCs (Figure 6B) [98]. Moreover, sHDL delivered multi-epitope antigens that induced broad T cell responses, resulting in enhanced tumor immunotherapy. Similarly, the combination of sHDL with adjuvants leads to a strong immune response resulting in effective tumor regression [99]. Further studies are needed to evaluate the co-delivery via different loading methods to determine the most effective targeting technique of immune cells and LNs. Studies are currently ongoing to investigate the ways to increase the effectiveness of immunotherapy by NPs depending on the formulation of adjuvant and antigen [100-102]. Groettrup et al. demonstrated that the immunogenicity of the encapsulated antigen and adjuvant in the same carrier yielded a higher immune response than those encapsulated in separate carriers [100, 103]. A biodegradable poly (D, L-lactide-co-glycolide) carrier was designed to co-deliver CpG ODNs or poly I:C with OVA antigen. Although both co-delivery methods using same or separate carrier enhanced T cell immunity, encapsulation within the same carrier strengthened the cytotoxic T lymphocyte response and further enhanced the cytokine secretion. Conversely, another recent study showed that separately loaded adjuvant and antigen resulted in greater or similar effects of immune therapy compared with similar carriers [102]. The CpG adjuvant was encapsulated into virus-like particles (VLPs), and p33 antigen was chemically conjugated on the same or separate VLPs. Delivering CpG and p33 in separate VLPs yielded greater CD8+ T cell responses compared with those delivered by the same VLPs. Collectively, NP-based drug delivery systems facilitates co-delivery of adjuvant and antigen either in a single or separate particle although the potency of immune response induced is still disputed. In addition, the therapeutic efficacy of the vaccine varied depending on the loading method of adjuvant and antigen, which should be considered in the design of a carrier for co-delivery.
Recently, the effect of physicochemical properties, especially the size of adjuvant-containing carriers on immune activation has attracted great attention in vaccine development. In addition, adjuvant density on the carrier surface and particle shape have been investigated [22, 92, 104-106]. Nanotechnology can be used to alter the shape of carriers and the density of adjuvants on the carrier. Physicochemical properties of carriers with adjuvants affect the targeting to dendritic cells (DCs) and modulation of DCs [107, 108]. During shape modification, alum-based adjuvants are commonly used in vaccines to stimulate the innate immune response [109-111]. In order to improve the adjuvant effect of alum, the immunological effect according to physicochemical properties was studied by Xia's group using synthetic γ-phase aluminum oxyhydroxide NPs (γ-ALOOH) in the form of nanoscale plates, polyhedra and various rod sizes [95]. In this report, ALOOH nanoplates and nanopolyhedra showed a lower level of cytokine expression than commercial alum, but ALOOH nanorods boosting the immune responses compared with the commercial alum. ALOOH nanorods enhanced NOD-, LRR- and pyrin domain-containing protein 3 (NLRP3) inflammasome activation and enhanced the release of pro-inflammatory cytokine IL-1β. The nanorods of smaller hydrodynamic size showed few in vivo immune responses among the various nanorods investigated. Notably, in another study, Niikura et al. demonstrated that the size and shape of particle influenced the adjuvant and immune responses depending on the injection method [112]. During intranasal administration, the shape of gold nanorods attached to adjuvant enhanced the immune response compared with spherical nanorods without inducing excessive inflammatory responses. However, the subcutaneous administration did not significantly affect the immune response according to the shape of gold NPs. The effect of carrier shape should be considered along with the effect of carrier size and route of administration. The effect of adjuvant density on particles has been investigated to optimize immunotherapy outcomes [92, 113]. Seder's group designed and evaluated various particles to enhance vaccine efficacy using TLR-7/8 agonist as an adjuvant [92]. The TLR agonist can be used to selectively stimulate leukocytes including APCs and DCs to enhance antigen-specific T cell immune response. Increasing the density of TLR-7/8 agonist on polymer carrier strongly stimulated CD4+ and CD8+ T cell immunity more than the lower density of adjuvant. Furthermore, TLR-7/8 agonists conjugated with polymer particles were markedly absorbed by DCs compared with the same total dose of adjuvant on the small molecule or polymer coil. Increasing the uptake of TLR-7/8 agonists conjugated with polymer particle by DCs enhanced the cytokine secretion in draining LNs. Interestingly, Roy et al. designed four types of pathogen-like particles (PLPs) based on the density of CpG adjuvant (maximum or low density) and the size of PLPs (microparticle or NP) [113]. DCs treated with a maximum density of CpG effectively increased the pro-inflammatory IL12p70 cytokine secretion, and also enhanced the amount of IFN-γ produced in CD4+ T cells that were co-cultured with DCs treated with PLPs at a maximum CpG density. In addition, IL4 secretion of CD4+T cells tended to decrease inversely with increasing concentration of CpG. IFN-γ is associated with Th1 differentiation, and IL4 is linked to Th2 differentiation. In addition, particle size skews the immune response. Micro-sized PLPs potentially stimulate pro-inflammatory cytokine release from DCs, resulting in skewing to Th1. Conversely, nano-sized PLPs can simultaneously skew immune response to either Th1 or Th2 depending on the CpG concentration on the particles. Accordingly, CpG density and particle size can potentially program DCs, polarizing immune responses into either Th1 or Th2. Based on these studies, it is apparent that biophysical effects of adjuvant on the particles can regulate the immune system and dictate the type of immune response. Therefore, biophysical attributes such as size or shape of the carriers and ligand density in particle design should be considered during the development of an effective cancer vaccine.
Recently, various strategies using NP-based delivery system have been developed to deliver adjuvant and antigen for effective antigen cross-presentation and enhanced immune response. NP-based antigen/adjuvant delivery system improved the preclinical outcomes compared with conventional soluble adjuvant/antigen treatment by enhancing target specificity and delivery efficiency. Furthermore, appropriate modification of biophysical properties of NPs facilitated delivery of adjuvant/antigen via various routes of administration, which improved the immune response. However, the mechanisms underlying the changes in immune system based on the biophysical properties of antigen-presenting NPs are still unclear. Furthermore, the physicochemical properties of NPs need to be optimized to avoid unintended polarization of immune cells. Finally, a scale-up manufacture and personalized design of adjuvant/antigen using NPs can enhance their potential therapeutic efficacy for clinical application.
5. Perspective and Conclusion
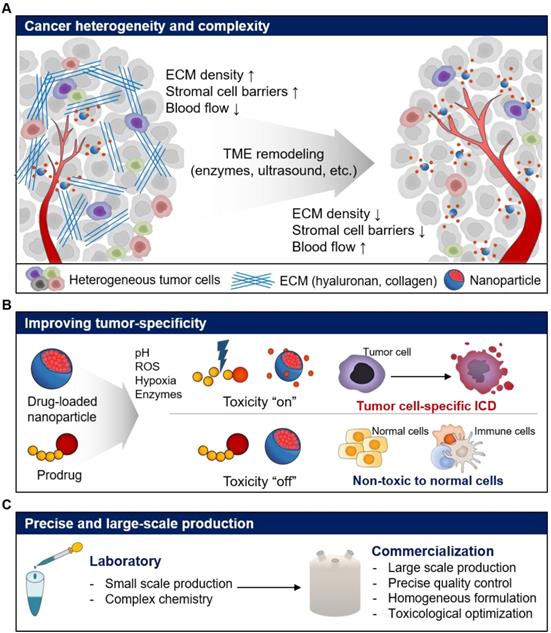
In this review, we discussed the different issues for repurposing of current NP-based drug delivery systems for cancer immunotherapy. Recent advances in NP-based drug delivery systems have enhanced the delivery of immunotherapeutics such as antigens, adjuvants, immune checkpoint inhibitors, cytokines, and cytotoxic anticancer agents by repurposing the current NP-based drug delivery systems, resulting in improved anticancer immune response (Table 1). However, there are a number of limitations and challenges, which need to be overcome, to repurpose NP-based drug delivery systems for the delivery of immunotherapeutics to tumors (Table 2). Inherent properties of tumor, such as complexity and heterogeneity, can reduce the efficiency of NP-based drug delivery [114]. The complex TME such as poor blood flow, stromal cell barriers, and dense ECM can interfere with tissue penetration of NPs, leading to reduced delivery efficiency. Furthermore, the targeting efficiency of NP-based drug delivery systems might vary between tumors due to the heterogeneous TME. Therefore, the delivery challenges need to be addressed to achieve the desired therapeutic efficacy of repurposed NP-based drug delivery systems (Figure 7A). Several groups reported that ECM remodeling strategy using enzymes such as hyaluronidase, collagenase, bromelain and matrix metalloproteinase, which degrade ECM in the tumor tissue, may enhance the delivery and tissue permeability of anticancer drugs and NPs [115-121]. For example, PEGylated form of recombinant human hyaluronidase (PEGPH20), which removes hyaluronan from the ECM of solid tumors showed a significant increase in microvessels and reduced interstitial fluid pressure [122]. In combination with anticancer drug, gemcitabine, PEGPH20 significantly inhibited the tumor progression and incidence of metastasis in a phase I clinical study [123]. Treatment of patients with hyaluronan high (HA-high) metastatic pancreatic ductal adenocarcinoma with a combination of PEGPH20 and pembrolizumab is also in phase II clinical study. Cheng et al. found that recombinant PH20-embedded HPEG-PH20 NPs showed deep tissue penetration in 4T1 breast tumor [124]. Notably, HPEG-PH20 NPs degraded HA matrix in tumor tissue, resulting in deep tissue delivery of DOX. Finally, HPEG-PH20 NPs prolonged the life span of tumor-bearing mice via successful inhibition of tumor growth. Therefore, versatile combinations and designs that can overcome these hurdles are needed to repurpose the current NP-based drug delivery systems for cancer immunotherapy.
To maximize the efficacy and minimize the toxicity in cancer immunotherapy, the target specificity and PK/PD of immunotherapeutics need to be carefully modulated, especially when cytotoxic drugs are used. Current NP-based drug delivery systems have shown potential for improved anticancer effect in recent decades. However, poor efficiency of drug encapsulation, innate toxicity and unintended release of cytotoxic drugs have reduced the therapeutic efficacy and resulted in adverse side effects [125, 126]. To overcome these limitations and induce ICD using cytotoxic drugs, the TME including enzymes, pH, reactive oxygen species (ROS), and hypoxic conditions can be used to design tumor cell-specific cytotoxic drugs (Figure 7B) [127-130]. For example, we designed cathepsin B-specific carrier-free prodrug NP (FRRG-DOX) using cathepsin B-cleavable peptide (Phe-Arg-Arg-Gly; FRRG) and doxorubicin for cancer targeting therapy [17]. FRRG-DOX exhibited reduced toxicity in normal cells, but activated toxicity in tumor cells, which over-expressed cathepsin B. Furthermore, FRRG-DOX formed NPs without carriers, showing successful tumor-targeting and tumor-specific toxicity via EPR effect in vivo. We expect that repurposing tumor-specific prodrug designs can be used to induce tumor cell-specific ICD as well as chemotherapy resulting in improving antitumor immunity.
Summarization of three categories of recent advances in NP-based delivery system for cancer immunotherapy
| Category | Agent | Cell type | Average size | Composition | Method | Ref |
|---|---|---|---|---|---|---|
| ICD-inducing cytotoxic NPs | DTX | Glioblastoma cell (HF2303) | 10-12 nm | ApoAI mimetic peptide, phospholipids and CpG | Self-assembly by nanodisc | [37] |
| DOX | Breast cancer cell (EMT6) | 170 nm | polymer-lipid based manganese dioxide NPs | Encapsulation | [43] | |
| DOX | Melanoma cell (B16F10) | 250 nm | matrix metalloproteinase sensitive peptide (CPLGLAGG) | Self-assembly with hyaluronic acid | [51] | |
| Oxaliplatin | Pancreatic ductal adenocarcinoma (KPC) | 80 nm | mesoporous silica NP with indoximod | Encapsulation | [53] | |
| PTX | Breast cancer cell (4T1) | 170 nm | Nano-formulation Exosome with PTX | Loading into exosome | [56] | |
| DOX | Isolate lymphocyte | 105 nm | c(RGDyK)-liposomes | Encapsulation | [58] | |
| Cytokines and cytokine-like immune modulators NPs | IL-2 | Melanoma cell (B16F10) | 80 nm | PEGylated liposomes with anti-CD137 | Surface-conjugated NP | [46] |
| TRAIL | Colon adenocarcinoma (COLO 205) | 100-140 nm | PEGylated liposomes with TRAIL | Surface-conjugated NP | [69] | |
| Peptide loaded MHC and anti-CD19 | Lymphoblast cell (Raji) | 50 nm | Anti-mouse IgG1 Microbeads with pepMHC and anti-CD19 | Surface-conjugated NP | [75] | |
| Anti-4-1BB and anti-PD-L1 | Melanoma cell (B16 SIY) | 80 nm | Antibiotin-coated iron-dextran with anti-4-1BB and anti-PD-L1 | Surface-conjugated NP | [76] | |
| Anti-HER2 and CRT | Breast cancer cell (E0771) | 45 nm | Carboxylated polystyrene NP with anti HER2 and CRT | Surface-conjugated NP | [77] | |
| M1 macrophages | Fibroblast cell (CT26) | 190 nm | M1NVs and anti-PD-L1 | Cell-derived nanovesicles | [78] | |
| TRAIL and E-selectin adhesion receptor | Colon adenocarcinoma (COLO 205) | 118 nm | Multilamellar liposomes (PC and Chol) | Surface-conjugated NP | [81] | |
| Stimulatory ligands | Jurkat T cells | 500 nm | anti-CD3 and anti-CD28 antibody | Conjugation with azide-functionalized silica NP | [83] | |
| NPs for adjuvant delivery | layered double hydroxide (LDH) | Melanoma cell (B16F10) | 140-150 nm | Tyrosinase-related protein 2 (Trp2) peptide | Adsorption and loading into LDH NP | [94] |
| Alum | Human THP-1 myeloid cell | 93-957 nm | γ-phase aluminum oxyhydroxide (γ-AlOOH) | Various size and shape of NP | [95] | |
| Poly I:C | Lymphoma cell (EG7-OVA) | 20-70 nm | Poly(γ-glutamic acid), OVA | Self-assembly and electrostatic adsorption | [96] | |
| CpG | Lymphoma cell (EL4 and EG7-OVA) | 10-20 nm | Albumin binding nanocomplexes, antigens (CSIINFEKL, Trp2, and Adpgk) | Chemical conjugation and self-assembly | [97] | |
| CpG | Melanoma cell (B16F10) | 10 nm | Phospholipids, ApoA1-mimetic peptides, antigens (SIINFEKL, CSSSIINFEKL) | Self-assembly by nanodiscs | [98] |
AlOOH: Aluminum hydroxide, ApoAI: Apolipoprotein-I, Chol: Cholesterol, CRT: Cell surface-exposed calreticulin, DOX: Doxorubicin, DTX: Docetaxel, ICD: Immunogenic cell death, IL-2: Interleukin 2, M1NVs: M1 macrophage-derived nanovesicles, NPs: Nanoparticles, OVA: Ovalbumin, PC: Phosphatidylcholine, pepMHC: Major histocompatibility complex binding peptide, PTX: paclitaxel, TRAIL: TNF-related apoptosis-inducing ligand.
Expected challenges and perspectives for the repurposing of current nanoparticle-based drug delivery systems for cancer immunotherapy. (A) Repurposing strategies to overcome cancer heterogeneity and complexity for improved drug delivery efficiency. (B) Repurposing strategies improved tumor specificity of immunotherapeutics by activating immune responses with fewer side effects. (C) Repurposing strategies for precise and large-scale commercial production of immunotherapeutics.
Merits and demerits, and research opportunities of three categories of recent advances in NP-based delivery system for cancer immunotherapy
| Category | Summary | Merits | Demerits or research opportunities |
|---|---|---|---|
| ICD-inducing cytotoxic NPs | - Additional synergic mechanisms by ICD-inducing NPs may be used in current cancer immunotherapy to improve therapeutic efficacy - Cytotoxic NPs modify immunosuppressive conditions in tumors by facilitating immunogenic antigens and enhancing ICD to potentiate cancer immunotherapy | - Maximal therapeutic efficacy with low toxicity - Available with conventional cytotoxic agents - Established in many preclinical and clinical tests - They could potentiate other cancer immunotherapies - A number of clinically available cytotoxic drugs for ICD | - Risk of cytotoxic drug-induced systemic toxicity - Unpredictable therapeutic efficacy of cytotoxic drugs - Need for further clinical studies - Potential need for adjuvant or immune-stimulating agents |
| Cytokines and cytokine-like immune modulators NPs | - Delivery of cytokine and cytokine-like immune modulators to target cells using NPs can overcome short half-lives and low stability in blood- NPs selectively trigger cancer cell apoptosis and elicit immune response in leukocytes - Multifaceted and Janus NPs provide simultaneous targeting of tumor and immune cells to boost anticancer activity | - Delivery cytokines to target sites with minimal adverse and off-target effects - Reduced cytokine or cytokine-like immune modulator degradation in blood- Easy surface presentation of various immune modulators - Enhanced anti-tumor immune response | - Further mechanistic and translational studies - Risk of cytokine or immune modulator resistance - Limited utilization in personalized immunotherapy |
| NPs for adjuvant delivery | - Co-delivery of adjuvant and antigen using NPs for effective immunotherapy - Adjuvant delivery with cancer antigens using NPs more effective in immune cells of LNs resulting in antigen cross-presentation | - Effective co-loading and delivery of adjuvant and antigen to LNs - Various administration routes - Easy modulation of adjuvant biophysical properties | - Limited utilization in personalized immunotherapy - Unintended polarization may occur via adjuvant modification - Weak stability for large-scale manufacture |
ICD: Immunogenic cell death, LNs: Lymph nodes, NPs: Nanoparticles
Beyond the tumor specificity of cytotoxic drugs, effective modulation of PK and the PD of the immune modulators including cytokines and immune checkpoint inhibitors is also needed to optimize the activation of immune responses in TME. Recombinant cytokines were approved for cancer immunotherapy; however, the short half-life of cytokines and cytokine release syndrome following high-dose bolus injection reduces the therapeutic efficacy [131]. Also, cytokine treatment induces autoimmune reaction in normal tissue by promoting regulatory T cell survival and T cell death [132]. Therefore, various approaches that improve target specificity and drug release are needed to repurpose the current NP-based drug delivery systems for optimal therapeutic efficacy and minimal toxicity. Finally, a relatively complex synthesis and quality control system is needed for the large-scale production of current NP-based drug delivery systems and their successful commercialization (Figure 7C). Thus, a precisely controlled and relatively simple chemistry suitable for large-scale production is needed to repurpose the current NP-based drug delivery systems. For example, a number of cancer vaccines with a synergistic effect have been developed. However, their clinical outcomes are unsatisfactory due to suboptimal conditions for large-scale manufacture, quality control, delivery efficiency of adjuvants and antigens to lymphoid organs, leading to weak immunostimulation and immune tolerance [61, 133, 134]. To address these issues, Chen's group designed nanovaccines (AlbiVax) that form nanocomplexes (Albumin/AlbiVax) with endogenous albumin in vivo [97]. In combination with anti-PD-1 or Abraxane, AlbiVax significantly inhibited tumor progression. Compared with conventional designs of nanovaccine, the endogenous albumin may have advantages including i) relatively simple and well-defined, chemistry-based, exogenous vaccine design to facilitate large-scale manufacture under stringent quality control, ii) antitumor immunity based on precise and effective delivery of nanovaccines to the target immune cells, resulting in enhanced delivery efficiency via albumin endocytosis, in which albumin receptors such as monocyte, DCs, and macrophages are highly expressed, and iii) increased PK of nanovaccines in vivo due to the albumin half-life in vivo [87]. This study suggested a strategy to repurpose the current NP-based drug delivery systems for clinical translation, and relatively simple and well-defined large-scale production. A number of NPs related to cancer immunotherapy are currently in clinical trials after large-scale manufacturing process. Representative NPs in the translational stages are Lipovaxin-MM (Avg. 240 nm, IFN-γ with liposome) for malignant melanoma treatment (phase I, NCT01052142), Oncoquest-L (IL-2 with proteo-liposome) for lymphoma treatment (phase II, NCT02194751), and CYT004-MelQbG10 (A-type CpG with virus-like NP) for malignant melanoma treatment. Taken together, various NPs in immunotherapy have shown promise for further clinical application.
In this review, we summarized the recent advances for repurposing current NP-based drug delivery systems for cancer immunotherapy. Various designs and strategies of NP-based drug delivery systems were utilized to elicit antitumor immunity. Furthermore, the current limitations and challenges associated with NP-based drug delivery systems have been discussed, and future directions for nanomedicines to overcome these hurdles have been discussed. Using versatile strategies to overcome the current limitations, we expect that repurposing the current NP-based drug delivery systems provides an opportunity for successful cancer immunotherapy and further clinical applications.
Abbreviations
AlbiAg: albumin-binding antigen; AlbiCpG: albumin-binding adjuvant; AlbiVax: albumin-binding vaccines; Alum: aluminum salts; APCs: antigen-presenting cells; CpG ODNs: CpG oligodeoxynucleotides; CRT: cell surface-exposed calreticulin; EPR: enhanced permeability and retention; FDA: Food and Drug Administration; HA: hyaluronic acid; HES-D-IL-2: IL-2 cytokines conjugated on the surface of hydroxyethyl starch; HMGB1: high mobility groups protein B1; ICD: immunogenic cell death; IDO: indoleamine 2,3-dioxygenase; IL-2: interleukin-2; IND: indoximod; INF: interferon; LDH: layered double hydroxide; NLRP3: NOD-, LRR- and pyrin domain-containing protein 3; LNs: lymph nodes; LPS: lipopolysaccharide; MMP: matrix metalloproteinase; MSNPs: mesoporous silica nanoparticles; NP: nanoparticle; OVA: ovalbumin; OX: oxaliplatin; PD: pharmacodynamics; PD-L1: programmed cell death protein-ligand 1; PK: pharmacokinetics; poly I:C: polyinosinic:polycytidylic acid; PTX: paclitaxel; SVNPs: synthetic vaccine nanoparticles; Th1: T helper cell type 1; Th2: T helper cell type 2; TILs: tumor-infiltrating lymphocytes; TLR: toll-like receptor; TME: tumor microenvironment; TNF: tumor necrosis factor; TRAIL: tumor necrosis factor related apoptosis-inducing ligand.
Acknowledgements
This work was supported by grants from the National Research Foundation (NRF) of Korea, funded by the Ministry of Science (NRF-2019R1A2C3006283), KU-KIST Graduate School of Converging Science and Technology (Korea University) and the Intramural Research Program of KIST.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Darvin P, Toor SM, Sasidharan Nair V, Elkord E. Immune checkpoint inhibitors: recent progress and potential biomarkers. Exp Mol Med. 2018;50:165
2. Park YJ, Kuen DS, Chung Y. Future prospects of immune checkpoint blockade in cancer: from response prediction to overcoming resistance. Exp Mol Med. 2018;50:109
3. Wei SC, Duffy CR, Allison JP. Fundamental Mechanisms of Immune Checkpoint Blockade Therapy. Cancer Discov. 2018;8:1069-86
4. Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R. et al. Safety and Tumor Responses with Lambrolizumab (Anti-PD-1) in Melanoma. N Engl J Med. 2013;369:134-44
5. Graziani G, Tentori L, Navarra P. Ipilimumab: A novel immunostimulatory monoclonal antibody for the treatment of cancer. Pharmacol Res. 2012;65:9-22
6. Zimmer L, Goldinger SM, Hofmann L, Loquai C, Ugurel S, Thomas I. et al. Neurological, respiratory, musculoskeletal, cardiac and ocular side-effects of anti-PD-1 therapy. Eur J Cancer. 2016;60:210-25
7. Amos SM, Duong CP, Westwood JA, Ritchie DS, Junghans RP, Darcy PK. et al. Autoimmunity associated with immunotherapy of cancer. Blood. 2011;118:499-509
8. Winer A, Bodor JN, Borghaei H. Identifying and managing the adverse effects of immune checkpoint blockade. J Thorac Dis. 2018;10:S480-9
9. Liu X, Li Y, Sun X, Muftuoglu Y, Wang B, Yu T. et al. Powerful anti-colon cancer effect of modified nanoparticle-mediated IL-15 immunogene therapy through activation of the host immune system. Theranostics. 2018;8:3490-503
10. Kong M, Tang J, Qiao Q, Wu T, Qi Y, Tan S. et al. Biodegradable Hollow Mesoporous Silica Nanoparticles for Regulating Tumor Microenvironment and Enhancing Antitumor Efficiency. Theranostics. 2017;7:3276-92
11. He J, Duan S, Yu X, Qian Z, Zhou S, Zhang Z. et al. Folate-modified Chitosan Nanoparticles Containing the IP-10 Gene Enhance Melanoma-specific Cytotoxic CD8(+)CD28(+) T Lymphocyte Responses. Theranostics. 2016;6:752-61
12. Liu L, Chen Q, Ruan C, Chen X, Zhang Y, He X. et al. Platinum-Based Nanovectors Engineered with Immuno-Modulating Adjuvant for Inhibiting Tumor growth and Promoting Immunity. Theranostics. 2018;8:2974-87
13. Tong X, Wang ZT, Sun XL, Song JB, Jacobson O, Niu G. et al. Size Dependent Kinetics of Gold Nanorods in EPR Mediated Tumor Delivery. Theranostics. 2016;6:2039-51
14. Bazak R, Houri M, El Achy S, Kamel S, Refaat T. Cancer active targeting by nanoparticles: a comprehensive review of literature. J Cancer Res Clin Oncol. 2015;141:769-84
15. Gu ZL, Wang QJ, Shi YB, Huang Y, Zhang J, Zhang XK. et al. Nanotechnology-mediated immunochemotherapy combined with docetaxel and PD-L1 antibody increase therapeutic effects and decrease systemic toxicity. J Control Release. 2018;286:369-80
16. Raave R, van Kuppevelt TH, Daamen WF. Chemotherapeutic drug delivery by tumoral extracellular matrix targeting. J Control Release. 2018;274:1-8
17. Shim MK, Park J, Yoon HY, Lee S, Um W, Kim JH. et al. Carrier-free nanoparticles of cathepsin B-cleavable peptide-conjugated doxorubicin prodrug for cancer targeting therapy. J Control Release. 2019;294:376-89
18. Gotwals P, Cameron S, Cipolletta D, Cremasco V, Crystal A, Hewes B. et al. Prospects for combining targeted and conventional cancer therapy with immunotherapy. Nat Rev Cancer. 2017;17:286-301
19. Zhu YQ, Feijen J, Zhong ZY. Dual-targeted nanomedicines for enhanced tumor treatment. Nano Today. 2018;18:65-85
20. Phung CD, Nguyen HT, Tran TH, Choi HG, Yong CS, Kim JO. Rational combination immunotherapeutic approaches for effective cancer treatment. J Control Release. 2019;294:114-30
21. Toy R, Roy K. Engineering nanoparticles to overcome barriers to immunotherapy. Bioeng Transl Med. 2016;1:47-62
22. Kumar S, Anselmo AC, Banerjee A, Zakrewsky M, Mitragotri S. Shape and size-dependent immune response to antigen-carrying nanoparticles. J Control Release. 2015;220:141-8
23. Hubbell JA, Thomas SN, Swartz MA. Materials engineering for immunomodulation. Nature. 2009;462:449-60
24. Wang J, Tao W, Chen X, Farokhzad OC, Liu G. Emerging Advances in Nanotheranostics with Intelligent Bioresponsive Systems. Theranostics. 2017;7:3915-9
25. Restifo NP, Smyth MJ, Snyder A. Acquired resistance to immunotherapy and future challenges. Nat Rev Cancer. 2016;16:121-6
26. Emens LA, Ascierto PA, Darcy PK, Demaria S, Eggermont AMM, Redmond WL. et al. Cancer immunotherapy: Opportunities and challenges in the rapidly evolving clinical landscape. Eur J Cancer. 2017;81:116-29
27. Galon J, Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discov. 2019;18:197-218
28. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF. et al. Safety, Activity, and Immune Correlates of Anti-PD-1 Antibody in Cancer. N Engl J Med. 2012;366:2443-54
29. Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJM, Robert L. et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515:568
30. Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH. et al. Survival, Durable Tumor Remission, and Long-Term Safety in Patients With Advanced Melanoma Receiving Nivolumab. J Clin Oncol. 2014;32:1020
31. Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L. et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015;372:2521-32
32. Pfirschke C, Engblom C, Rickelt S, Cortez-Retamozo V, Garris C, Pucci F. et al. Immunogenic Chemotherapy Sensitizes Tumors to Checkpoint Blockade Therapy. Immunity. 2016;44:343-54
33. Casares N, Pequignot MO, Tesniere A, Ghiringhelli F, Roux S, Chaput N. et al. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J Exp Med. 2005;202:1691-701
34. Bracci L, Schiavoni G, Sistigu A, Belardelli F. Immune-based mechanisms of cytotoxic chemotherapy: implications for the design of novel and rationale-based combined treatments against cancer. Cell Death Differ. 2014;21:15-25
35. Brown JS, Sundar R, Lopez J. Combining DNA damaging therapeutics with immunotherapy: more haste, less speed. Br J Cancer. 2018;118:312-24
36. Krysko DV, Garg AD, Kaczmarek A, Krysko O, Agostinis P, Vandenabeele P. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer. 2012;12:860-75
37. Kadiyala P, Li D, Nunez FM, Altshuler D, Doherty R, Kuai R. et al. High-Density Lipoprotein-Mimicking Nanodiscs for Chemo-immunotherapy against Glioblastoma Multiforme. ACS Nano. 2019;13:1365-84
38. Pusuluri A, Wu D, Mitragotri S. Immunological consequences of chemotherapy: Single drugs, combination therapies and nanoparticle-based treatments. J Control Release. 2019;305:130-54
39. Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 2000;65:271-84
40. Golombek SK, May JN, Theek B, Appold L, Drude N, Kiessling F. et al. Tumor targeting via EPR: Strategies to enhance patient responses. Adv Drug Deliver Rev. 2018;130:17-38
41. Dai YL, Xu C, Sun XL, Chen XY. Nanoparticle design strategies for enhanced anticancer therapy by exploiting the tumour microenvironment. Chem Soc Rev. 2017;46:3830-52
42. Zhang B, Hu Y, Pang Z. Modulating the Tumor Microenvironment to Enhance Tumor Nanomedicine Delivery. Front Pharmacol. 2017;8:952
43. Amini MA, Abbasi AZ, Cai P, Lip H, Gordijo CR, Li J. et al. Combining Tumor Microenvironment Modulating Nanoparticles with Doxorubicin to Enhance Chemotherapeutic Efficacy and Boost Antitumor Immunity. J Natl Cancer Inst. 2019;111:399-408
44. Zhang F, Stephan SB, Ene CI, Smith TT, Holland EC, Stephan MT. Nanoparticles That Reshape the Tumor Milieu Create a Therapeutic Window for Effective T-cell Therapy in Solid Malignancies. Cancer Res. 2018;78:3718-30
45. Miller MA, Zheng YR, Suresh GW, Pfirschke C, Zope H, Engblom C. et al. Tumour-associated macrophages act as a slow-release reservoir of nano-therapeutic Pt(IV) pro-drug. Nat Commun. 2015:6
46. Zhang Y, Li N, Suh H, Irvine DJ. Nanoparticle anchoring targets immune agonists to tumors enabling anti-cancer immunity without systemic toxicity. Nat Commun. 2018;9:6
47. Rodallec A, Sicard G, Fanciullino R, Benzekry S, Lacarelle B, Milano G. et al. Turning cold tumors into hot tumors: harnessing the potential of tumor immunity using nanoparticles. Expert Opin Drug Metab Toxicol. 2018;14:1139-47
48. Gao S, Yang D, Fang Y, Lin X, Jin X, Wang Q. et al. Engineering Nanoparticles for Targeted Remodeling of the Tumor Microenvironment to Improve Cancer Immunotherapy. Theranostics. 2019;9:126-51
49. Qin SY, Cheng YJ, Lei Q, Zhang AQ, Zhang XZ. Combinational strategy for high-performance cancer chemotherapy. Biomaterials. 2018;171:178-97
50. Kuai R, Yuan WM, Son S, Nam J, Xu Y, Fan YC. et al. Elimination of established tumors with nanodisc-based combination chemoimmunotherapy. Sci Adv. 2018:4
51. Gao F, Zhang C, Qiu WX, Dong X, Zheng DW, Wu W. et al. PD-1 Blockade for Improving the Antitumor Efficiency of Polymer-Doxorubicin Nanoprodrug. Small. 2018:14
52. Wu AA, Drake V, Huang HS, Chiu SC, Zheng L. Reprogramming the tumor microenvironment: tumor-induced immunosuppressive factors paralyze T cells. Oncoimmunology. 2015:4
53. Lu J, Liu X, Liao YP, Salazar F, Sun B, Jiang W. et al. Nano-enabled pancreas cancer immunotherapy using immunogenic cell death and reversing immunosuppression. Nat Commun. 2017;8:1811
54. Wang ZJ, Liu WH, Shi JY, Chen N, Fan CH. Nanoscale delivery systems for cancer immunotherapy. Mater Horiz. 2018;5:344-62
55. Yu G, Jung H, Kang YY, Mok H. Comparative evaluation of cell- and serum-derived exosomes to deliver immune stimulators to lymph nodes. Biomaterials. 2018;162:71-81
56. Wang PP, Wang HH, Huang QQ, Peng C, Yao L, Chen H. et al. Exosomes from M1-Polarized Macrophages Enhance Paclitaxel Antitumor Activity by Activating Macrophages-Mediated Inflammation. Theranostics. 2019;9:1714-27
57. Feng B, Zhou FY, Hou B, Wang DG, Wang TT, Fu YL. et al. Binary Cooperative Prodrug Nanoparticles Improve Immunotherapy by Synergistically Modulating Immune Tumor Microenvironment. Adv Mater. 2018:30
58. Wang XY, Wang H, Jiang K, Zhang YY, Zhan CY, Ying M. et al. Liposomes with cyclic RGD peptide motif triggers acute immune response in mice. J Control Release. 2019;293:201-14
59. Quesada JR, Hersh EM, Manning J, Reuben J, Keating M, Schnipper E. et al. Treatment of hairy cell leukemia with recombinant alpha-interferon. Blood. 1986;68:493-7
60. Rosenberg SA. IL-2: the first effective immunotherapy for human cancer. J Immunol. 2014;192:5451-8
61. Riley RS, June CH, Langer R, Mitchell MJ. Delivery technologies for cancer immunotherapy. Nat Rev Drug Discov. 2019;18:175-96
62. Lipiainen T, Peltoniemi M, Sarkhel S, Yrjonen T, Vuorela H, Urtti A. et al. Formulation and stability of cytokine therapeutics. J Pharm Sci. 2015;104:307-26
63. Torrealba D, Parra D, Seras-Franzoso J, Vallejos-Vidal E, Yero D, Gibert I. et al. Nanostructured recombinant cytokines: A highly stable alternative to short-lived prophylactics. Biomaterials. 2016;107:102-14
64. Kedar E, Gur H, Babai I, Samira S, Even-Chen S, Barenholz Y. Delivery of cytokines by liposomes: hematopoietic and immunomodulatory activity of interleukin-2 encapsulated in conventional liposomes and in long-circulating liposomes. J Immunother. 2000;23:131-45
65. Segura S, Gamazo C, Irache JM, Espuelas S. Gamma interferon loaded onto albumin nanoparticles: in vitro and in vivo activities against Brucella abortus. Antimicrob Agents Chemother. 2007;51:1310-4
66. Christian DA, Hunter CA. Particle-mediated delivery of cytokines for immunotherapy. Immunotherapy. 2012;4:425-41
67. Guimaraes PPG, Gaglione S, Sewastianik T, Carrasco RD, Langer R, Mitchell MJ. Nanoparticles for Immune Cytokine TRAIL-Based Cancer Therapy. ACS Nano. 2018;12:912-31
68. Frick SU, Domogalla MP, Baier G, Wurm FR, Mailander V, Landfester K. et al. Interleukin-2 Functionalized Nanocapsules for T Cell-Based Immunotherapy. ACS Nano. 2016;10:9216-26
69. Nair PM, Flores H, Gogineni A, Marsters S, Lawrence DA, Kelley RF. et al. Enhancing the antitumor efficacy of a cell-surface death ligand by covalent membrane display. Proc Natl Acad Sci U S A. 2015;112:5679-84
70. Hallaj-Nezhadi S, Lotfipour F, Dass C. Nanoparticle-mediated interleukin-12 cancer gene therapy. J Pharm Pharm Sci. 2010;13:472-85
71. Park K. Facing the Truth about Nanotechnology in Drug Delivery. ACS Nano. 2013;7:7442-7
72. Kedar E, Palgi O, Golod G, Babai I, Barenholz Y. Delivery of cytokines by liposomes.3. Liposome-encapsulated GM-CSF and TNF-alpha show improved pharmacokinetics and biological activity and reduced toxicity in mice. J Immunother. 1997;20:180-93
73. Byeon HJ, Min SY, Kim I, Lee ES, Oh KT, Shin BS. et al. Human serum albumin-TRAIL conjugate for the treatment of rheumatoid arthritis. Bioconjug Chem. 2014;25:2212-21
74. Lattuada M, Hatton TA. Synthesis, properties and applications of Janus nanoparticles. Nano Today. 2011;6:286-308
75. Schutz C, Varela JC, Perica K, Haupt C, Oelke M, Schneck JP. Antigen-specific T cell Redirectors: a nanoparticle based approach for redirecting T cells. Oncotarget. 2016;7:68503-12
76. Kosmides AK, Sidhom JW, Fraser A, Bessell CA, Schneck JP. Dual Targeting Nanoparticle Stimulates the Immune System To Inhibit Tumor Growth. ACS Nano. 2017;11:5417-29
77. Yuan H, Jiang W, von Roemeling CA, Qie Y, Liu X, Chen Y. et al. Multivalent bi-specific nanobioconjugate engager for targeted cancer immunotherapy. Nat Nanotechnol. 2017;12:763-9
78. Choo YW, Kang M, Kim HY, Han J, Kang S, Lee JR. et al. M1 Macrophage-Derived Nanovesicles Potentiate the Anticancer Efficacy of Immune Checkpoint Inhibitors. ACS Nano. 2018;12:8977-93
79. Noh YW, Kim SY, Kim JE, Kim S, Ryu J, Kim I. et al. Multifaceted Immunomodulatory Nanoliposomes: Reshaping Tumors into Vaccines for Enhanced Cancer Immunotherapy. Adv Funct Mater. 2017:27
80. Park W, Heo YJ, Han DK. New opportunities for nanoparticles in cancer immunotherapy. Biomater Res. 2018;22:24
81. Mitchell MJ, Wayne E, Rana K, Schaffer CB, King MR. TRAIL-coated leukocytes that kill cancer cells in the circulation. Proc Natl Acad Sci U S A. 2014;111:930-5
82. Zhang Y, Huang K, Lin J, Huang P. Janus nanoparticles in cancer diagnosis, therapy and theranostics. Biomater Sci. 2019;7:1262-75
83. Lee K, Yu Y. Janus Nanoparticles for T Cell Activation: Clustering Ligands to Enhance Stimulation. J Mater Chem B. 2017;5:4410-5
84. Lee K, Zhang L, Yi Y, Wang X, Yu Y. Rupture of Lipid Membranes Induced by Amphiphilic Janus Nanoparticles. ACS Nano. 2018;12:3646-57
85. Zhang L, Zhang M, Zhou L, Han Q, Chen X, Li S. et al. Dual drug delivery and sequential release by amphiphilic Janus nanoparticles for liver cancer theranostics. Biomaterials. 2018;181:113-25
86. Wang L, Shi C, Wang X, Guo D, Duncan TM, Luo J. Zwitterionic Janus Dendrimer with distinct functional disparity for enhanced protein delivery. Biomaterials. 2019;215:119233
87. Zhu G, Zhang F, Ni Q, Niu G, Chen X. Efficient Nanovaccine Delivery in Cancer Immunotherapy. ACS Nano. 2017;11:2387-92
88. Hu Z, Ott PA, Wu CJ. Towards personalized, tumour-specific, therapeutic vaccines for cancer. Nat Rev Immunol. 2018;18:168-82
89. Wilson JT, Keller S, Manganiello MJ, Cheng C, Lee CC, Opara C. et al. pH-Responsive nanoparticle vaccines for dual-delivery of antigens and immunostimulatory oligonucleotides. ACS Nano. 2013;7:3912-25
90. Zhang ZP, Tongchusak S, Mizukami Y, Kang YJ, Ioji T, Touma M. et al. Induction of anti-tumor cytotoxic T cell responses through PLGA-nanoparticle mediated antigen delivery. Biomaterials. 2011;32:3666-78
91. Fan Y, Kuai R, Xu Y, Ochyl LJ, Irvine DJ, Moon JJ. Immunogenic Cell Death Amplified by Co-localized Adjuvant Delivery for Cancer Immunotherapy. Nano Lett. 2017;17:7387-93
92. Lynn GM, Laga R, Darrah PA, Ishizuka AS, Balaci AJ, Dulcey AE. et al. In vivo characterization of the physicochemical properties of polymer-linked TLR agonists that enhance vaccine immunogenicity. Nat Biotechnol. 2015;33:1201-10
93. Fan Y, Moon JJ. Nanoparticle Drug Delivery Systems Designed to Improve Cancer Vaccines and Immunotherapy. Vaccines (Basel). 2015;3:662-85
94. Zhang L-x, Xie X-x, Liu D-q, Xu ZP, Liu R-t. Efficient co-delivery of neo-epitopes using dispersion-stable layered double hydroxide nanoparticles for enhanced melanoma immunotherapy. Biomaterials. 2018;174:54-66
95. Sun B, Ji Z, Liao YP, Wang M, Wang X, Dong J. et al. Engineering an effective immune adjuvant by designed control of shape and crystallinity of aluminum oxyhydroxide nanoparticles. ACS Nano. 2013;7:10834-49
96. Kim SY, Noh YW, Kang TH, Kim JE, Kim S, Um SH. et al. Synthetic vaccine nanoparticles target to lymph node triggering enhanced innate and adaptive antitumor immunity. Biomaterials. 2017;130:56-66
97. Zhu GZ, Lynn GM, Jacobson O, Chen K, Liu Y, Zhang HM. et al. Albumin/vaccine nanocomplexes that assemble in vivo for combination cancer immunotherapy. Nat Commun. 2017:8
98. Kuai R, Ochyl LJ, Bahjat KS, Schwendeman A, Moon JJ. Designer vaccine nanodiscs for personalized cancer immunotherapy. Nat Mater. 2017;16:489-96
99. Lu F, Mosley YC, Carmichael B, Brown DD, HogenEsch H. Formulation of aluminum hydroxide adjuvant with TLR agonists poly(I:C) and CpG enhances the magnitude and avidity of the humoral immune response. Vaccine. 2019;37:1945-53
100. Schlosser E, Mueller M, Fischer S, Basta S, Busch DH, Gander B. et al. TLR ligands and antigen need to be coencapsulated into the same biodegradable microsphere for the generation of potent cytotoxic T lymphocyte responses. Vaccine. 2008;26:1626-37
101. Bode C, Zhao G, Steinhagen F, Kinjo T, Klinman DM. CpG DNA as a vaccine adjuvant. Expert Rev Vaccines. 2011;10:499-511
102. Mohsen MO, Gomes AC, Cabral-Miranda G, Krueger CC, Leoratti FM, Stein JV. et al. Delivering adjuvants and antigens in separate nanoparticles eliminates the need of physical linkage for effective vaccination. J Control Release. 2017;251:92-100
103. Mueller M, Reichardt W, Koerner J, Groettrup M. Coencapsulation of tumor lysate and CpG-ODN in PLGA-microspheres enables successful immunotherapy of prostate carcinoma in TRAMP mice. J Control Release. 2012;162:159-66
104. Niikura K, Matsunaga T, Suzuki T, Kobayashi S, Yamaguchi H, Orba Y. et al. Gold Nanoparticles as a Vaccine Platform: Influence of Size and Shape on Immunological Responses in vitro and in vivo. ACS Nano. 2013;7:3926-38
105. Reuter KG, Perry JL, Kim D, Luft JC, Liu R, DeSimone JM. Targeted PRINT Hydrogels: The Role of Nanoparticle Size and Ligand Density on Cell Association, Biodistribution, and Tumor Accumulation. Nano Lett. 2015;15:6371-8
106. Bandyopadhyay A, Fine RL, Demento S, Bockenstedt LK, Fahmy TM. The impact of nanoparticle ligand density on dendritic-cell targeted vaccines. Biomaterials. 2011;32:3094-105
107. Reddy ST, van der Vlies AJ, Simeoni E, Angeli V, Randolph GJ, O'Neil CP. et al. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat Biotechnol. 2007;25:1159-64
108. Hanson MC, Crespo MP, Abraham W, Moynihan KD, Szeto GL, Chen SH. et al. Nanoparticulate STING agonists are potent lymph node-targeted vaccine adjuvants. J Clin Invest. 2015;125:2532-46
109. Morefield GL, Sokolovska A, Jiang D, HogenEsch H, Robinson JP, Hem SL. Role of aluminum-containing adjuvants in antigen internalization by dendritic cells in vitro. Vaccine. 2005;23:1588-95
110. Hem SL, Hogenesch H. Relationship between physical and chemical properties of aluminum-containing adjuvants and immunopotentiation. Expert Rev Vaccines. 2007;6:685-98
111. Parween S, Gupta PK, Chauhan VS. Induction of humoral immune response against PfMSP-1(19) and PvMSP-1(19) using gold nanoparticles along with alum. Vaccine. 2011;29:2451-60
112. Tazaki T, Tabata K, Ainai A, Ohara Y, Kobayashi S, Ninomiya T. et al. Shape-dependent adjuvanticity of nanoparticle-conjugated RNA adjuvants for intranasal inactivated influenza vaccines. RSC Adv. 2018;8:16527-36
113. Leleux JA, Pradhan P, Roy K. Biophysical Attributes of CpG Presentation Control TLR9 Signaling to Differentially Polarize Systemic Immune Responses. Cell Rep. 2017;18:700-10
114. Wilhelm S, Tavares AJ, Dai Q, Ohta S, Audet J, Dvorak HF. et al. Analysis of nanoparticle delivery to tumours. Nat Rev Mater. 2016;1:16014
115. Yhee JY, Jeon S, Yoon HY, Shim MK, Ko H, Min J. et al. Effects of tumor microenvironments on targeted delivery of glycol chitosan nanoparticles. J Control Release. 2017;267:223-31
116. Hong Y, Nam GH, Koh E, Jeon S, Kim GB, Jeong C. et al. Exosome as a Vehicle for Delivery of Membrane Protein Therapeutics, PH20, for Enhanced Tumor Penetration and Antitumor Efficacy. Adv Funct Mater. 2018;28:1703074
117. Cui M, Naczynski DJ, Zevon M, Griffith CK, Sheihet L, Poventud-Fuentes I. et al. Multifunctional Albumin Nanoparticles As Combination Drug Carriers for Intra-Tumoral Chemotherapy. Adv Healthc Mater. 2013;2:1236-45
118. Raeesi V, Chan WC. Improving nanoparticle diffusion through tumor collagen matrix by photo-thermal gold nanorods. Nanoscale. 2016;8:12524-30
119. Parodi A, Haddix SG, Taghipour N, Scaria S, Taraballi F, Cevenini A. et al. Bromelain surface modification increases the diffusion of silica nanoparticles in the tumor extracellular matrix. ACS Nano. 2014;8:9874-83
120. Callmann CE, Barback CV, Thompson MP, Hall DJ, Mattrey RF, Gianneschi NC. Therapeutic Enzyme-Responsive Nanoparticles for Targeted Delivery and Accumulation in Tumors. Adv Mater. 2015;27:4611-5
121. Ruan S, Cao X, Cun X, Hu G, Zhou Y, Zhang Y. et al. Matrix metalloproteinase-sensitive size-shrinkable nanoparticles for deep tumor penetration and pH triggered doxorubicin release. Biomaterials. 2015;60:100-10
122. Thompson CB, Shepard HM, O'Connor PM, Kadhim S, Jiang P, Osgood RJ. et al. Enzymatic depletion of tumor hyaluronan induces antitumor responses in preclinical animal models. Mol Cancer Ther. 2010;9:3052-64
123. Infante JR, Korn RL, Rosen LS, LoRusso P, Dychter SS, Zhu J. et al. Phase 1 trials of PEGylated recombinant human hyaluronidase PH20 in patients with advanced solid tumours. Br J Cancer. 2017;118:153
124. Zhou H, Fan Z, Deng J, Lemons PK, Arhontoulis DC, Bowne WB. et al. Hyaluronidase Embedded in Nanocarrier PEG Shell for Enhanced Tumor Penetration and Highly Efficient Antitumor Efficacy. Nano Lett. 2016;16:3268-77
125. Sharma A, Madhunapantula SV, Robertson GP. Toxicological considerations when creating nanoparticle-based drugs and drug delivery systems. Expert Opin Drug Metab Toxicol. 2012;8:47-69
126. Hare JI, Lammers T, Ashford MB, Puri S, Storm G, Barry ST. Challenges and strategies in anti-cancer nanomedicine development: An industry perspective. Adv Drug Deliv Rev. 2017;108:25-38
127. Tao W, Ji X, Zhu X, Li L, Wang J, Zhang Y. et al. Two-Dimensional Antimonene-Based Photonic Nanomedicine for Cancer Theranostics. Adv Mater. 2018;30:1802061
128. Ye M, Han Y, Tang J, Piao Y, Liu X, Zhou Z. et al. A Tumor-Specific Cascade Amplification Drug Release Nanoparticle for Overcoming Multidrug Resistance in Cancers. Adv Mater. 2017;29:1702342
129. Prijovich ZM, Burnouf P-A, Chou H-C, Huang P-T, Chen K-C, Cheng T-L. et al. Synthesis and Antitumor Properties of BQC-Glucuronide, a Camptothecin Prodrug for Selective Tumor Activation. Mol Pharm. 2016;13:1242-50
130. Kim HS, Sharma A, Ren WX, Han J, Kim JS. COX-2 Inhibition mediated anti-angiogenic activatable prodrug potentiates cancer therapy in preclinical models. Biomaterials. 2018;185:63-72
131. Lee S, Margolin K. Cytokines in cancer immunotherapy. Cancers (Basel). 2011;3:3856-93
132. Francis DM, Thomas SN. Progress and opportunities for enhancing the delivery and efficacy of checkpoint inhibitors for cancer immunotherapy. Adv Drug Deliv Rev. 2017;114:33-42
133. Irvine DJ, Swartz MA, Szeto GL. Engineering synthetic vaccines using cues from natural immunity. Nat Mater. 2013;12:978-90
134. Zhao L, Seth A, Wibowo N, Zhao CX, Mitter N, Yu C. et al. Nanoparticle vaccines. Vaccine. 2014;32:327-37
Author contact
![]() Corresponding author: (E-mail) kimre.kr
Corresponding author: (E-mail) kimre.kr
 Global reach, higher impact
Global reach, higher impact