13.3
Impact Factor
Theranostics 2019; 9(25):7792-7806. doi:10.7150/thno.35486 This issue Cite
Review
Opportunities and Challenges for Antibodies against Intracellular Antigens
1. Nanobody Research Center, Guangxi Medical University, Nanning, Guangxi, 530021, China
2. School of Preclinical Medicine, Guangxi Medical University, Nanning, Guangxi, 530021, China
3. School of Stomatology, Guangxi Medical University, Nanning, Guangxi, 530021, China
*These authors contributed equally to this work.
Received 2019-4-4; Accepted 2019-7-26; Published 2019-10-15
Abstract
Therapeutic antibodies are one most significant advances in immunotherapy, the development of antibodies against disease-associated MHC-peptide complexes led to the introduction of TCR-like antibodies. TCR-like antibodies combine the recognition of intracellular proteins with the therapeutic potency and versatility of monoclonal antibodies (mAb), offering an unparalleled opportunity to expand the repertoire of therapeutic antibodies available to treat diseases like cancer. This review details the current state of TCR-like antibodies and describes their production, mechanisms as well as their applications. In addition, it presents an insight on the challenges that they must overcome in order to become commercially and clinically validated.
Keywords: peptide, TCR-like antibody, MHC-peptide complex, CAR-T cell therapy
Introduction
Cancer has caused tens of millions of deaths globally, making it the second leading cause of human deaths [1-3]. In the ongoing war against cancer, immuno-oncology (I-O), a product of the many breakthroughs and discoveries in immunology and cancer therapy, has raised our hopes of improving cancer survival [4]. In addition, I-O and its advancements were named the 2013's scientific breakthrough of the year by “science” [5]. During the last few years, I-O based monoclonal antibody therapies have progressed at a rapid pace, the Food and Drug Administration (FDA) has approved over 50 monoclonal antibodies targeting PD-1, CTLA-4, CD30, CD20 and so on, which gave rise to now commercially and clinically available drugs such as Rituximab and Trastuzumab [6-8]. The biological and pharmacological properties of monoclonal antibodies make them attractive to the researchers [9].
Regardless of the approach, the target antigens are mostly extracellular proteins [10, 11]. This is partly due to the antibodies' high molecular weight that prevents them from crossing the cellular membrane and thus target intracellular antigens. Given that majority of the tumor-associated antigen (TAA) proteins produced by a cancer cell are produced intracellularly, the number of externally expressed tumor antigens is limited. What' s more, majority of the proteins identified as specific tumor markers are intracellularly localized such as the case of WT1, which is regarded as the most promising among the 75 representative target antigens [12, 13]. Altogether, intracellular proteins may provide an untapped reservoir of potential therapeutic targets.
Many efforts have been made to target intracellular antigens; these strategies can be divided into two broad approaches. The first approach is to target the antigens that are normally intracellular but become externalized in exceptional situations, as in the case of heat-shock protein 70, heat-shock protein 90, phosphatase of regenerating liver 3 (PRL-3) and gp75 [14-17]. Zeng and colleagues focused on PRL-3, a cancer-related phosphatase which is undetectable in most normal human tissues but over-expressed in 85% of gastric cancers, and developed a humanized anti-PRL-3 antibody. The second approach is to engineer the antibodies or specific fragments to penetrate the cells or to express antibodies using a gene therapy approach. Some of these approaches include the usage of viral vectors, liposomes, nanoparticles and the fusion of peptides and antibodies [18-22].
Furthermore, intracellular proteins are degraded by the proteasomes to form short peptides of specific lengths which are normally 8-10 amino acids long. These peptides are then presented on the cell surface of the cancer cells in the context of major histocompatibility complex class I (MHC-I) molecules, forming various MHC-peptide antigens that can be recognized by T cells [23-25]. Since the MAGE-1 gene was reported to encode a human tumor antigen recognized by T cells, molecular identification and characterization of novel tumor-associated antigens (TAAs) has expanded rapidly [26-31]. To date, there are hundreds of identified MHC-peptide antigens, which can be used for the development of diagnostic methods and targeting therapy for cancer [32-34].
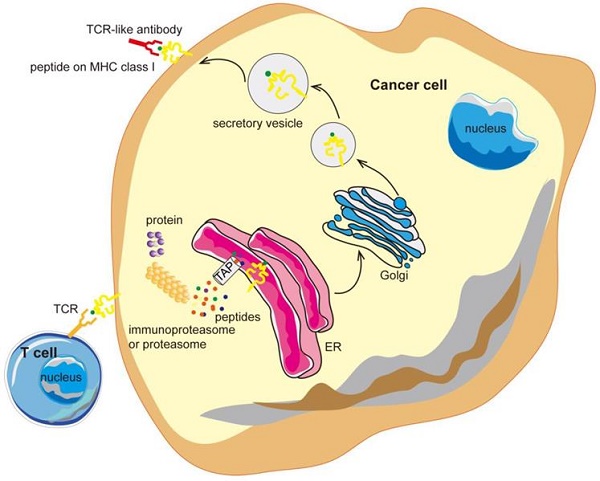
Antibodies targeting the MHC-peptide complexes are known as the T cell receptor mimic (TCRm) monoclonal antibodies (mAbs) or T-cell receptor (TCR) -like antibodies. TCR-like antibodies can combine the recognition of intracellular proteins (analogous to that of TCRs but with higher affinity) and the therapeutic potency as well as the versatility of mAbs [35, 36]. TCR-like antibodies are redefining the selection of suitable targets in cancer therapy and may open the door to a new realm of antibody therapy, with promising clinical benefits (Figure 1).
TCR-like antibodies binding to specific MHC-peptide complexes on a cancer cell. Intracellular proteins can be degraded by the proteasome and processed into peptides that are then presented on the cell surface in the context of MHC class I molecules. TCR-like antibodies can specifically target cancer cells exhibiting specific MHC-peptide complexes on their surface. MHC, major histocompatibility complex; ER, endoplasmic reticulum.
Production of TCR-like antibodies
During the last decades there has been significant progress in the development of TCR-like antibodies, and several research groups have been able to generate TCR-like antibodies directed to a growing repertoire of cancer. The traditional production manner of TCR-like antibodies is hybridoma technology. The first and most important step using conventional hybridoma is to obtain purified recombinant MHC-peptide complexes that are recognized by a T cell [37, 38]. These complexes are prepared by the means of bacterial expression (usually Escherichia coli.) to generate inclusion bodies comprising the extracellular domain of the heavy chain of human leukocyte antigen (HLA) and β2-microglobulin. Thence, the HLA heavy chain and 2-microglobulin inclusion bodies are refolded in the presence of the desired HLA-restricted peptides in vitro [39-41]. The refolding must be processed in the right conformation, as demonstrated by structural and functional studies, then these complexes can be used for downstream applications [42].
At first, researchers employing traditional hybridoma technology used antigen-presenting cells (APCs) presenting strong immunogenic peptides in their MHC complex as immunogens but not the purified recombinant MHC-peptide complexes [43, 44]. The peptide-specific, MHC-restricted antibodies using the above-mentioned technique are quite rare even under optimal conditions; given that one to three out of 1000 growth positive clones could produce antibodies of the requested specificity [45-50]. Although the attempts to improve this technique have failed several times, numerous research groups have used recombinant MHC-peptide complexes for the isolation of TCR-like antibodies and have been successful in using conventional hybridoma technology [51-55].
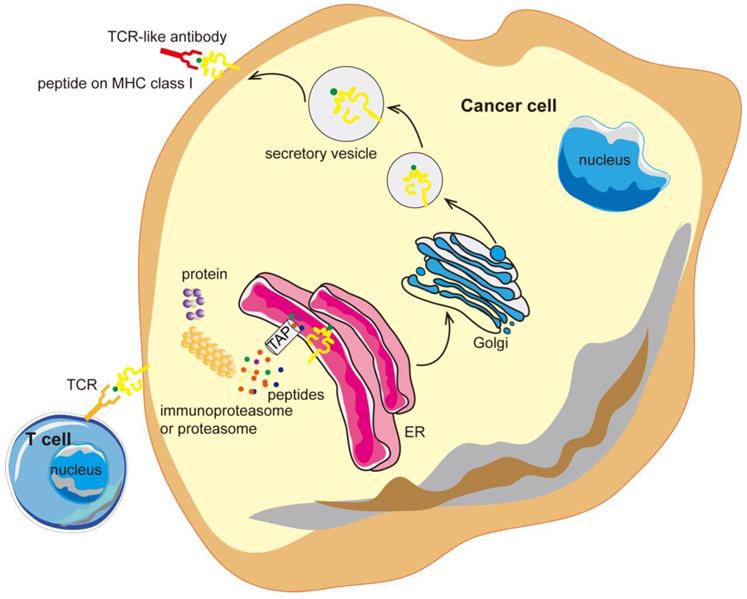
In the mid-1990s, it was shown that phage display technology could also be used to isolate antibodies (Figure 2). The pioneering work by Andersen and colleagues demonstrated that phage-display could be used as a tool to isolate antibodies with unique specificity, furthermore, subsequent works consecutively proved phage-display technology to be a promising way to isolate antibodies [56]. The first step of phage-display is to generate antibody libraries exposed as fusion proteins on the surface of phage particles. These libraries are called the naïve libraries, and each phage particle in the naïve libraries displays a unique antibody. Phage particles carrying specific antibodies are purified by repeated rounds of selection, and then TCR-like antibodies (scFv/Fab) are isolated from large naïve human phage-display libraries [57-60]. The phage-based approach can be consistently applied to isolate recombinant antibodies with the requested specificity, providing new means for TCR-like antibodies production.
The affinity of TCR-like antibodies isolated from a naïve phage-display library is not always sufficient for therapeutic purposes. Similar to hybridoma technology, many efforts have been made to improve the affinity of the TCR-like antibodies, second-generation libraries generated by different affinity-maturation strategies are used for the isolation of the TCR-like antibodies. Chames and colleagues isolated an 18-fold affinity TCR-like Fab (the VH-VL hybrid clone Hyb3) directed to the cancer T-cell peptide HLA-A1-MAGE-A1, using Fab G8 as the platform for the construction of two randomized libraries: L chain shuffling library and H chain complementarity determining region 3 mutated library [61]. Renner and colleagues have achieved the 20-fold affinity improvement of a new TCR-like Fab to the HLA-A-0201-NY-ESO-1 peptide using a second-generation Fab library. This Fab library is based on Fab 2M4E5 in which they randomized residues at positions that could optimize peptide interaction to improve their affinity, without changing the key residues responsible for the binding of the complex antigen [62]. It was also reported that using transgenic mice expressing the desired human MHC allele on a murine MHC knocked out background would increase the probability of isolating a rare TCR-like antibody [63].
Most of the TCR-like antibodies published works have used phage display for antibody production [64-68]. The major advantage of the phage display approach is the high selection power of the desired antigens due that the process is being achieved within a relatively short time, conversely the generation of TCR-like antibodies using hybridoma technology is less efficient and relatively more time consuming [69]. TCR-like antibodies isolated using hybridoma technology were reported to have higher binding affinity compared to the moderate average affinity of TCR-like antibodies isolated from the naïve phage display libraries [70, 71]. Therefore, using hybridoma technology could have a tendency for isolating antibodies with high-affinity binding to the MHC-peptide complexes. The antibodies produced by hybridoma technology are bivalent IgG isotype antibodies while antibodies produced by phage display are either scFv or Fab fragments. IgG antibodies are more stable and have a superior affinity due that antibodies undergo multiple antigen challenges and affinity maturation in vivo. Antibodies in the monovalent form have reduced avidity (functional affinity) and increased turnover rates, which are undesirable when targeting peptides on tumor-associated MHC-peptide complexes. Although monovalent forms are useful reagents for a variety of TCR-like applications, their reduced binding strength is a great limitation. To overcome this limitation, it is reported that Fab or scFv-tetramers or transformer of IgG isotype can improve the binding avidity [72-75].
Generation of antibody libraries and selection of the TCR-like antibodies. (A) Diverse repertoires can be obtained from the rearranged V-gene segments which are derived either from naïve or activated B cells subsequent to immunization or infection or human V-gene segments rearranged in vitro (synthetic repertoires). The assembled scFv/Fab repertoires are then cloned into a phagemid vector in order to be expressed on the surface of the phage as single-chain scFv or Fab antibody libraries and then the phage library is incubated with the desired target cells. (B) After incubation, there are two types of the phages. The unbound phages are removed through washing and the bound phages are eluted and propagated in E. coli. The bound phages are then used for further rounds of selection to get the specific binders. Fab, fragment antigen binding; scFv, single-chain variable fragment.
Production techniques for TCR-like antibodies are not shared, and most phage display libraries are proprietary. Tao Dao and colleagues have discovered a fully human “T cell receptor-like” monoclonal antibody (mAb) ESK1, specific for the WT1 RMF peptide/HLA-A0201 complex. The antibody ESK1, developed in collaboration with Memorial Sloan Kettering Cancer Center (MSKCC) and Eureka Therapeutics, is now patented. Eureka Therapeutics, a pioneer in the development of TCR-like antibodies; currently works with different companies to develop TCR-like antibodies against different intracellular proteins. Bringing the developed TCR-like antibodies into clinical trials as soon as possible is one of the most notable challenges faced by all researchers in this area.
TCR-like antibodies to date
Over the past 20 years, there has been an increase in the production of TCR-like antibodies. Initially most of the target peptides of these antibodies were derived from viruses; however, with the introductions of TCR-like antibodies, new key targets for the treatment of cancer have been discovered [76-83]. Having two different production strategies, TCR-like antibodies can be bivalent IgG isotype antibodies produced through hybridoma technology or the scFv or Fab fragments produced through phage display. Regarding IgG isotype antibodies, most of them were produced by four laboratories. Weidanz's research laboratory engineered RL4B/3.2G1 targeting HLA-A2-restricted GVL peptide derived from the parent protein hCGβ, 1B8 targeting HLA-A2-restricted KIF peptide derived from parent protein Her2/neu in 2006, 1B10/3F9 targeting HLA-A2-restricted GVL peptide/TMT peptide derived from parent protein hCGβ in 2008, RL6A targeting HLA-A2-restricted YLL peptide derived from parent protein p68 in 2010, and RL21A targeting HLA-A2-restricted MIF19-27 peptide in 2011 [84-88]. Banham's research laboratory engineered T1-29D/T1-84C/T1-116C targeting HLA-A2-restricted RMP peptide derived from parent protein p53, and T2-108A/T2-2A/T2-116A targeting HLA-A2-restricted GLA peptide derived from parent protein p53 in 2017 [89, 90]. Molldrem's research laboratory engineered a TCR-like antibody 8F4 targeting HLA-A2-restricted VLQ peptide derived from parent protein Proteinase3 in 2011 [91, 92]. Scheinberg's research laboratory engineered the antibody ESK1 targeting HLA-A2-restricted RMF peptide derived from parent protein WT1 in 2013, and its Fc enhanced form ESKM in 2014 [93, 94].
The scFv or Fab fragments produced through phage display are not complete IgG isotype antibodies and they cannot recruit components of the immune system for cytotoxic effects through antibody-dependent cell-mediated cytotoxicity (ADCC) and complement dependent cytotoxicity (CDC). Literatures regarding TCR-like antibodies like scFv or Fab fragments published to date can be divided in three groups.
The first group focuses on the selection and characterization of the Fab/scFv fragments, researchers used naked TCR-like antibodies to target cells. Patrick Chames and colleagues engineered a fully human Fab fragment Fab-G8 directed against the HLA-A1-MAGE-A1 complex by selection from a large naïve phage-antibody library in 2000, posteriorly they enhanced TCR-like antibody Fab-Hyb3 by selection from a second-generation library in 2002 [61, 95]. Galit Denkberg and collaborators engineered Fab fragments like 1A7 from a large nonimmune repertoire of phage Fab Abs in 2002 [96]. Avital Lev and collaborators finished the isolation of some human antibodies with antigen-specificity, MHC-restricted specificity of T cells binding with HLA-A2 complexes which display the specific hTERT-derived peptide [97]. Christoph Renner's laboratory described the selection and characterization of Fab fragments recognizing the NY-ESO-1157-165 peptide in the HLA-A*0201 context [98]. Renner's laboratory also selected Fab antibodies binding to the HLA-A2-restricted EAA or ELA peptide derived from the parent protein Melan-A [99].
Similarly, articles from the second group describe the selection and characterization of the Fab/scFv fragments, however in this case researchers modified the structure of the TCR-like antibodies. Galit Denkberg and colleagues reported for the first time the fusion of the TCR-like antibody gene to a truncated form of Pseudomonas exotoxin A to form a recombinant immunotoxin [63, 100]. Cyril J. Cohen and colleagues reported the production of fluorescent tetramerized Fabs to directly visualize and quantitate the specific HLA-A2/MUC1-D6 peptide on the surface of tumor cells [101]. David A. Scheinberg's laboratory selected a ScFv fragment that is specific for the WT1 RMF peptide/HLA-A*0201 complex found on many human cancers, they also engineered the scFv fragment to a full length human monoclonal antibody to target cancer cells [93]. The three before mentioned reports are the main representatives of this type of literatures and there are also some papers reporting similar work [102-104].
The third group describes the selection and characterization of Fab/scFv fragments, in this articles researchers describe the development of TCR-like antibodies into a CAR based approach on the TCR-like antibody of a high-affinity antibody that recognizes the complex antigen composed of the MHC molecule and a peptide derived from the antigen protein. David A. Scheinberg's laboratory selected a scFv fragment that is specific for the WT1 RMF peptide/HLA-A*0201 complex and then created a TCRm CAR against WT1 utilizing the previously described scFv which demonstrated effective in vitro/vivo efficacy [105]. WT1 is over-expressed in numerous hematological malignancies like acute myeloid leukemia (AML), as well as in many solid malignancies such as ovarian cancer, thus the created WT1 TCRm CAR T-cell approach allows for the application of a single CAR to a wider array of malignancies [106-108]. Hong Liu and colleagues developed ET1402L1, a fully human antibody that selectively binds to AFP158-166 (AFP158) peptide presented by HLA-A*0201, then engineered this antibody into a second generation CAR, demonstrating that CAR-T cell immunotherapy targeting intracellular/secreted solid tumor antigens like AFP can elicit potent anti-tumor responses [109-112]. Similar works targeting different peptides presented by HLA-A molecules have been reported [113-118].
Mechanisms of the TCR-like antibody function
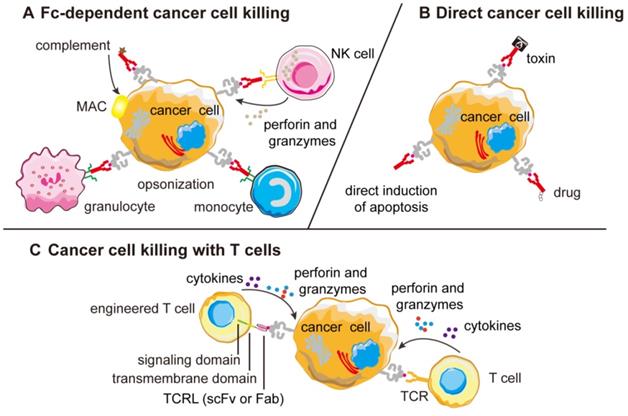
Similar to monoclonal antibodies that target specific tumor antigens, naked TCR-like antibodies can also be used to mediate ADCC and CDC, which are also called the Fc-dependent mechanisms of mAb. RL4B and other naked TCR-like antibodies have been shown to induce CDC mechanisms in vitro, which are induced by the binding of the complement proteins to the Fc region of therapeutic TCR-like antibodies, however most of the naked TCR-like antibodies have been proved to induce ADCC mechanisms in vitro [119-122]. The exact mechanism of ADCC varies depending on the type of immune effector cells that are activated. Activated NK cells secrete perforin and granzyme B, which are taken up by the target cells and result in their lysis, while monocytes and macrophages are capable of secreting cytotoxic factors like TNF and reactive oxygen intermediates [123-125].
Naked TCR-like antibodies like RL4B can also to deliver a direct apoptotic signal to cancer cells in a mechanism involving JNK activation and the caspase-dependent pathway, directly killing the target cells [126]. Similar to the Fc-independent killing mechanism, immunoconjugates such as immunotoxins and immuno-drugs are able to directly kill the target cells. The targeting moiety of the immunoconjugates can be a Fab or a scFv fragment composed of the variable domains VH and VL which are covalently linked through a peptide linker [127-132]. Immunotoxins or immuno-drugs' mechanisms of action allows them to directly kill cancer cells, conferring them a clinical benefit in the treatment of patients who may not respond to agents that require a fully functioning immune system.
In adoptive T-cell transfer therapeutic approaches like CAR-T cell therapy, autologous T cells are isolated, expanded and engineered in vitro and re-infused to patients [133-135]. TCR-like antibodies are responsible for recognition, while cytotoxic T-cell signaling moiety FcεRIγ chain is responsible for the initiation of tumor-specific killing activities and cytokines release [136, 137]. The engineered T cells were found to specifically bind MHC-peptide complexes on target cells, leading to the production of cytokines and induction of cytolysis (Figure 3).
The applications of TCR-like antibodies
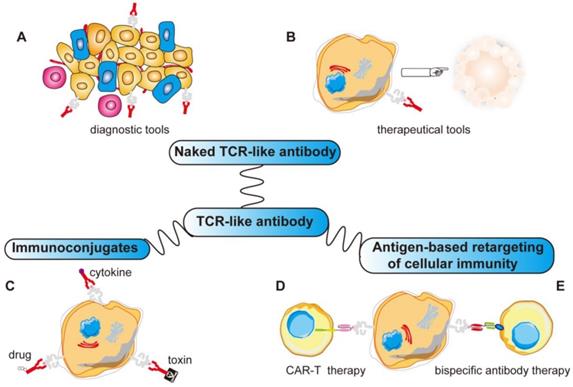
TCR-like antibodies can be used to directly visualize the presence of MHC-peptide complexes by standard methods such as flow cytometry [138-140]. Since TCR-like antibodies can provide novel data regarding antigen presentation in various cells, TCR-like antibodies can be used to analyze immunotherapy-based approaches by determining the alterations in MHC-peptide complexes expression on cells before, during and after the therapies, this could also provide new powerful means to study the structure-function relationships in the MHC-peptide context [141-149]. Since the density of a particular MHC-peptide complex on tumor cells is expected to be low compared to peptide-pulsed or transfected APCs, TCR-like antibodies were engineered to make tetramers, with directly tagged fluorescent probes [150].
More importantly, TCR-like antibodies present new opportunities for use as targeting moieties for various antibody-based immunotherapeutic approaches because of their exquisite specificity towards a very precise and unique human tumor antigen. This includes using such antibodies to construct recombinant immunotoxins/drugs, fusion with cytokine molecules, bispecific antibody therapy and for CAR-T therapy [151-160]. With more applications yet to be explored, TCR-like antibodies promise to be as useful as monoclonal antibodies (Figure 4).
Choosing the ideal target
Tumor associated antigens can be classified into three categories: cancer testis antigens and oncofetal antigens, differentiation antigens and over-expressed antigens. Cancer testis antigens and oncofetal antigens like MAGE, WT1 as well as alpha-fetoprotein are expressed in a wild range of different cancer cells, but limited in normal tissues except for embryonic cells or germ cells [161-164]. Differentiation antigens are restrictively expressed in limited cell lineages, such as gp100, Tyrosinase, and MART-1 [165-168]. Over-expressed antigens are normal proteins, but over-expressed or amplified in cancer cells, such as PR1, Her2/neu, PSA, EGFR [169-176]. They are all potential cancer targets, and they have their own pros and cons. Targeting the differentiation antigens like KRAS G12V/D is the most conceptually interesting approach as it can kill specific cancer cells without injuring normal cells, nevertheless they are also difficult to rationalize for therapeutic drug development [177]. Targeting the cancer testis antigens and over-expressed antigens like NY-ESO-1 and Her2/neu could also be a more general strategy, although the expression on the normal healthy tissues must be considered [178, 179].
Mechanism of action of TCR-like antibodies against cancer cells. (A) Most naked TCR-like antibodies induce CDC or ADCC mechanisms that are Fc-dependent and the ADCC mechanism can be different among different effector cells. (B) Naked TCR-like antibodies can also induce apoptosis mechanism. When fused to toxins or drugs, the fusion protein can kill the tumor cells directly. (C) T-cells engineered to display TCR-like antibodies as receptors can re-direct cytotoxic T cells against cancer cells forming lytic immunological synapse. CDC, complement-dependent cytotoxicity; MAC, membrane attack complex; ADCC, antibody-dependent cell mediated cytotoxicity; Fab, fragment antigen binding; scFv, single-chain variable fragment; MHC, major histocompatibility complex; TCRL, T-cell receptor-like.
The process of proteins expression and the presentation of short peptides on MHC are well described. However, the specific rules that govern which protein peptides are ultimately presented on the cell surface are poorly understood and therefore not fully predictable. Further identification of the presented peptides is an empirical process [180, 181]. In cancer, the discovery of these peptides mostly emerged from the observation that cancer cells express antigens which can be recognized by cytotoxic T-lymphocytes (CTLs) derived from patients [182-189]. Some peptides may be generated but have low affinity to MHC, while other peptides may have high affinity to MHC molecule, but never reach the cell surface due to improper processing [190]. Hence, many potentially interesting targets are not available as MHC-presented cell-surface peptides.
Databases such as the Cancer Genome Atlas can be used to identify mutated protein sequences that can be used as potential targets, but current methods to identify peptides presented by cancer cells on surface MHC may lack the required sensitivity, and thus are able to identify only a limited number of possible antigens [191, 192]. Therefore, some peptides that are likely to be TCR-like antibody targets, may not be detected. cDNA expression cloning is used as the original technique for isolating tumor antigens recognized by CD8+ T cells, but with this method is difficult to determine MHC restriction for unique antigens. Posteriorly, SEREX (serological analysis of recombinant cDNA expression libraries), cDNA expression cloning using serum IgG Ab from cancer patients, was developed. But RNA expression levels do not correlate with that of protein expression, in other words, RNA expression levels do not concord with peptide presentation levels. Therefore, mass spectrometry is the direct way to identify peptides presented by cancer cells on surface MHC, and it has been the consensus in the field [193].
Various applications of TCR-like antibodies. Fab/scFv fragments with MHC restricted specificity obtained by phage display can be used in different ways: (A, B) Directly to target the specific MHC-peptide complexes as diagnostic tools or therapeutic tools. (C) Fused to a drug/toxin/cytokine to form immunoconjugates. (D) Fused as a signaling moiety to genetically retargeting T cells toward cancer cells. (E) Reformated as bispecific antibody binding simultaneously a MHC-peptide complex and a receptor expressed by effector cells (CD3 on T cells). ADCC, antibody-dependent cellular cytotoxicity; CDC, complement-dependent cytotoxicity; Fab, fragment antigen binding; scFv, single-chain variable fragment; MHC, major histocompatibility complex.
Although both TCR-like antibodies and traditional antibodies can bind to antigens, the binding sites and methods are different. Traditional mAbs bind to conformational antigens, whereas TCR-like antibodies recognize complex antigens composed of MHC molecules with embedded short peptides. For a given haplotype, the MHC component is invariant, and the embedded peptides can come from the millions of sequences encoded in the exome. Most TCR-like antibodies have been shown to bind only a few residues of their target linear peptide [194-196]. This suggests that TCR-like antibodies may theoretically have many off-target peptides that share the same residues at major contacts, but differ on other positions. For instance, the therapeutic TCR-like antibody ESK1 only binds to complex antigens composed of HLA-A*0201 amino acids in addition to 3-5 N-terminal residues of the WT1-derived peptide 9-mer. Exchange of the C-terminal amino acid of the target peptide still allows binding of ESK1 TCR-like antibody. On the contrary, a TCR-like antibody targeting the cancer-testis antigen PRAME was shown to bind the C-terminus of the full-length sequence as well as TCR-like antibody targeting the tumor-associated antigen PR1 that was shown to depend heavily on one residue of the PR1-peptide [197, 198].
Enhancing peptide density
The peptide density of TCR-like antibody targets has been reported to hold only 100-1,000 sites per cell, which is significantly lower than some reported peptide densities of conventional monoclonal antibody whose cell surface targets are 20,000-500,000 sites per cell. The density of TCR-like antibody cell surface sites is positively correlated with the killing effect of TCR-like antibodies on target cells, so a key factor that needs to be considered when choosing a target antigen for TCR-like antibody therapy include the peptide density on the cell surface. The levels of protein expression and presentation, HLA levels, protein half-life, levels of MHC-peptide complex presentation all dictate TCR-like target peptide density.
A sufficient amount of protein must be translated to facilitate peptide processing. For example, the hypomethylating agent Decitabine can significantly increase the expression of NY-ESO1 in patient's tumor biopsies [199]. Another methyltransferase inhibitor, 5-azacytidine, can also induce cancer-testis antigen-specific CTLs in patients, minimally affecting immune effector populations and function [200]. Protein stability also plays an important role, it is reported that defective ribosomal products (DRiPs) that can be degraded quickly constitute a large percentage of peptides presented on MHC, and they may accumulate due to errors in the process of transcription, translation, or protein folding. Short-lived proteins appear to be more likely than proteins with longer half-lives [201].
After the expression of the proteins, they are cleaved into random-sized peptides by proteasomes in the cytosol. Proteasomes are the major complex that degrades proteins into peptides, constitutive proteasome (CP) or the immuno-proteasome (IP) are the two major forms of proteasomes [202, 203]. Hence, modulation of proteasome expression by treatment with cytokines or proteasome inhibitors could enhance or eliminate the presentation of specific peptides [204]. To name some examples, Lactacystin is an organic compound naturally synthesized by bacteria of the genus Streptomyces, the influenza M1 58-66 peptide is more efficiently produced in the presence of Lactacystin, and a significant number of the cancer antigen MAGE-3 271-279 is presented by melanoma cells after the inhibition of Lactacystin [190, 205].
Posterior to degradation, these cytosolic peptides are then pumped into the endoplasmic reticulum through TAP, where they are trimmed by aminopeptidases, loaded onto MHC molecules, and transported to the cell surface. Peptide MHC loading is governed by the binding affinity of the MHC protein to the peptide. HLA molecules are not internalized readily, and it has been reported that up to 90% MHC class I down-regulation has been noted in several cancers [206]. Given all these obstacles, down regulation of the MHC class I expression prevents a properly processed peptide from reaching the cell surface. Using some agents to upregulate the MHC class I expression is necessary. MEK inhibitor PD98059 has been reported to increase MHC expression in esophageal and gastric cancers through the inhibition of the MAPK pathway [207]. Similarly, the EGFR inhibitor Erlotinib could induce increased MHC class I levels on patients treated with it [208]. In addition to small molecule modulation of the inhibitor, targeting associated protein molecules can also increase expression of MHC molecules. For example, β2 microglobulin (β2M) is often downregulated in cancers, using the modulators of β2M may have the possibility to increase target peptide presentation [209].
Future for the TCR-like antibodies
Based on the fact that T-cell receptor mimicking antibodies have not yet entered the clinic, several key factors have the potential to improve the development of TCR-like antibodies so that TCR-like antibodies can have the prospect of undertaking clinical studies and ultimately establish themselves as a type of cancer therapy.
Antibodies with the MHC-restricted specificity of T-cells are rare and lots of high-affinity, peptide-specific TCR-like antibodies have proven to be difficult to produce by either hybridoma approaches or phage display because B cells are not educated to be self-MHC restricted [210-214]. T cells are educated to recognize antigenic peptides presented in complex with MHC class I or II molecules through the alternating selection processes, while B-cells are not in this selection process. The creation of TCR-like antibodies is expensive and time consuming. In order to isolate fully high-affinity, peptide-specific human antibodies within a short period of time, the improvement of traditional production methods and even the creation of new production methods are necessary.
It is crucial to ensure that the TCR-like antibodies do not recognize the MHC-I alone, as this molecule is found on most nucleated cells, and it does not cross-react with other processed peptides, as TCR-like antibodies recognize only a few amino acid residues in the peptide, which means that other processed peptides possess the same amino acids at those positions [215, 216]. Therefore, the TCR-like antibody must be specific for the specific peptide-MHC complex. A clinical trial of an affinity-enhanced TCR, which targeted a MAGE-A3 peptide, was reported to cause two patient deaths [217]. It was discovered that the TCR also recognized a peptide on the unrelated protein titin that is expressed in cardiac tissue which was not observed in normal tissue screening and was not conserved in mice. The lack of suitable animal models to study whether TCR-like antibodies can target other cross-reactive peptides in vivo is a major problem disrupting its clinical applications.
One of the key limitations of TCR-like antibody therapy is the MHC restrictive nature of treatment, although it is vital to be able to recognize intracellular proteins. Most studies to date focused on the HLA-A*0201 haplotype, which is found in up to 40% of Caucasians and 10-20% of other ethnic groups around the world [218-220]. There are other dominant HLA alleles such as HLA-A*2402. Although TCR-like antibodies are HLA-restricted, it has been proposed that antibodies to three HLA alleles for a particular target antigen would cover over 96% of the world's population, it is reported that TCR-like antibodies bind multiple HLA-A*02 variants and not only the HLA-A*0201 subtype, suggesting that certain TCR-like antibodies could target a larger population of patients with a variety of HLA subtypes [221, 222]. Therefore, HLA-A restriction does not limit the treatment to a limited number of patients, and the use of HLA-restricted presentation peptides allows multiple antibodies to be designed for specific antigens for combination therapy that could achieve better results.
There are a lot of strategies to augment the therapeutic index of the TCR-like antibodies. In addition to increasing the expression of complex MHC-peptide antigens, the application of TCR-like antibodies to other therapeutic methods and the combination with other treatments are the future direction. TCR-like antibodies can serve as an ideal cancer targeting platform for the delivery of cytotoxic payloads specific to tumors such as potent drugs and toxins. TCR-like antibodies can also be engineered into bispecific T-cell engagers (BiTEs) and chimeric antigen receptors (CARs) for expression on cytotoxic T-cells [223-225]. CARs are single-chain variable fragment (scFv)-based receptors used to redirect T cells to recognize and lyse cancer cells. CARs would be advantageous in that they do not directly compete with the native TCR, and would further provide supportive co-stimulation to the transduced T cells [226]. In 2018, The FDA made history by approving the first gene therapy in the United States. Kymriah, a cell-based gene therapy, is approved in the United States for the treatment of patients up to 25 years of age with B-cell precursor ALL that is refractory or in second or later relapse. On October 18th of the same year, the FDA approved the Kite Yescarta (axicabtagene ciloleucel, KTE-C10) cell gene therapy for the treatment of diffuse large B-cell lymphoma [227, 228]. More importantly, Joseph A. Fraietta and colleagues reported that due to the proliferation of a single CAR-T cell, a patient with chronic lymphocytic leukemia (CLL) treated with CAR-T cells in 2013 was relieved and remains cancer-free for over 5 years, and that the CAR-T cells are still present in his immune system [229]. Due to the great therapeutic potential of CAR-T therapy, the application of TCR-like antibodies to CAR-T will make TCR-like antibodies the ideal companion for this role.
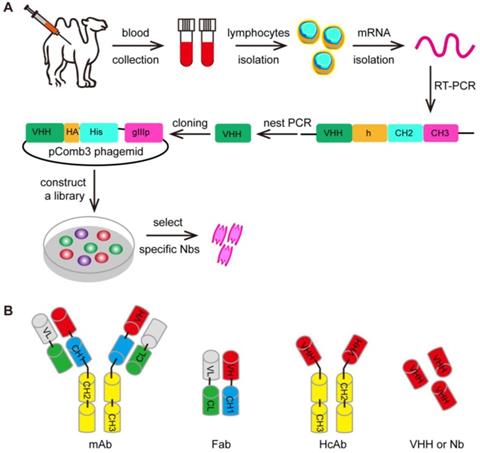
Furthermore, the variable (V) region domain can be used by its own to form a domain antibody called nanobody (Figure 5) [230]. These nanobodies can be engineered from the heavy-chain antibody (HcAb) derived from camelids (camel or llama) or cartilaginous fish (carpet or nurse sharks), whose immune systems were found to have evolved high-affinity V-like domains that do not require intramolecular disulfide bonds for stability [231-234]. The ability to specifically recognize unique epitopes with sub-nanomolar affinity have made nanobodies a useful class of biomolecules for medical research due to their various diagnostic and therapeutic applications. To name some recent examples, nanobodies have been employed as a cell re-targeting moiety in CAR-T cell therapy to target the extracellular antigens [235-237]. It has also reported that TCR-like CARs containing GPA7, a single-domain antibody (sdAb) specific for gp100 209-217/HLA-A2 complex, could mediate the enhanced cytotoxicity of transgenic T cells against HLA-A2-matched melanoma in vitro and in vivo [117, 238]. The variable (V) region domains represent the smallest format of the antibody that retains target specificity and it can replace traditional antibodies in a series of applications for cancer treatment.
The selection of the VHH. (A) The first step of the selection using phage-display technology is to generate VHH libraries. After repeated rounds of selection, the specific VHHs are isolated from the large libraries. (B) The various antibody formats: mAb (monoclonal antibody), Fab (fragment antigen binding), HcAb (camel heavy-chain antibody), VHH or Nb (nanobody).
Conclusion
Expanding the targeting repertoire to the intracellular proteome represented by the MHC molecules, the generation of antibodies that can target intracellular antigens offer unparalleled opportunities not only for optimizing cancer treatment but also for the development of new anticancer strategies. TCR-like antibodies transform the fine cellular specificity of the T-cell recognition into an antibody-based immunotherapeutic approach and also fit in with the growing field of personalized medicine. The vast new arrays of potential targets presented by the MHC molecules suggest that TCR-like antibodies will find an important place in our armamentarium, picturing a promising next step for immunotherapy.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Global regional, national life expectancy all-cause mortality, cause-specific mortality for 249 causes of death 1980-2015. a systematic analysis for the global burden of disease study 2015. Lancet. 2016;388:1459-544
2. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F. et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-32
3. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424
4. Hanahan D. Rethinking the war on cancer. Lancet. 2014;383:558-63
5. Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science. 2013;342:1432-3
6. Chiavenna SM, Jaworski JP, Vendrell A. State of the art in anti-cancer mAbs. J Biomed Sci. 2017;24:15
7. Cartron G, Dacheux L, Salles G, Solal-Celigny P, Bardos P, Colombat P. et al. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood. 2002;99:754-8
8. O'Donnell EK, Raje NS. New monoclonal antibodies on the horizon in multiple myeloma. Ther Adv Hematol. 2017;8:41-53
9. Weiner LM, Surana R, Wang S. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nat Rev Immunol. 2010;10:317-27
10. Hinrichs CS, Restifo NP. Reassessing target antigens for adoptive T-cell therapy. Nat Biotechnol. 2013;31:999-1008
11. Morello A, Sadelain M, Adusumilli PS. Mesothelin-targeted CARs:driving T cells to solid tumors. Cancer Discov. 2016;6:133-46
12. Weidle UH, Maisel D, Klostermann S, Schiller C, Weiss EH. Intracellular proteins displayed on the surface of tumor cells as targets for therapeutic intervention with antibody-related agents. Cancer Genomics Proteomics. 2011;8:49-63
13. Cheever MA, Allison JP, Ferris AS, Finn OJ, Hastings BM, Hecht TT. et al. The prioritization of cancer antigens: a national cancer institute pilot project for the acceleration of translational research. Clin Cancer Res. 2009;15:5323-37
14. Weidle UH, Maisel D, Brinkmann U, Tiefenthaler G. The translational potential for target validation and therapy using intracellular antibodies in oncology. Cancer Genomics Proteomics. 2013;10:239-50
15. Zininga T, Ramatsui L, Shonhai A. Heat shock proteins as immunomodulants. Molecules. 2018:23
16. Thura M, Al-Aidaroos AQO, Yong WP, Kono K, Gupta A, Lin YB. et al. PRL3-zumab, a first-in-class humanized antibody for cancer therapy. JCI Insight. 2016;1:e87607
17. Saha S, Bardelli A, Buckhaults P, Velculescu VE, Rago C, St Croix B. et al. A phosphatase associated with metastasis of colorectal cancer. Science. 2001;294:1343-6
18. Chatin B, Mével M, Devallière J, Dallet L, Haudebourg T, Peuziat P. et al. Liposome-based formulation for intracellular delivery of functional proteins. Mol Ther Nucleic Acids. 2015;4:e244
19. Peer D, Karp JM, Hong S, Farokhzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2:751-60
20. Danhier F, Ansorena E, Silva JM, Coco R, Le Breton A, Préat V. PLGA-based nanoparticles:an overview of biomedical applications. J Control Release. 2012;161:505-22
21. Zhao Y, Brown TL, Kohler H, Müller S. MTS-conjugated-antiactive caspase 3 antibodies inhibit actinomycin D-induced apoptosis. Apoptosis. 2003;8:631-7
22. Muller S, Zhao Y, Brown TL, Morgan AC, Kohler H. TransMabs: cell-penetrating antibodies, the next generation. Expert Opin Biol Ther. 2005;5:237-41
23. Malissen B, Bongrand P. Early T cell activation: integrating biochemical, structural, and biophysical cues. Annu Rev Immunol. 2015;33:539-61
24. Malissen B, Grégoire C, Malissen M, Roncagalli R. Integrative biology of T cell activation. Nat Immunol. 2014;15:790-7
25. Yewdell JW. Plumbing the sources of endogenous MHC class I peptide ligands. Curr Opin Immunol. 2007;19:79-86
26. van der Bruggen P, Traversari C, Chomez P, Lurquin C, De Plaen E, Van den Eynde B. et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science. 1991;254:1643-7
27. Kawakami Y, Eliyahu S, Delgado CH, Robbins PF, Sakaguchi K, Appella E. et al. Identification of a human melanoma antigen recognized by tumor-infiltrating lymphocytes associated with in vivo tumor rejection. Proc Natl Acad Sci U S A. 1994;91:6458-62
28. Kawakami Y, Robbins PF, Wang X, Tupesis JP, Parkhurst MR, Kang X. et al. Identification of new melanoma epitopes on melanosomal proteins recognized by tumor infiltrating T lymphocytes restricted by HLA-A1,-A2, and -A3 alleles. J Immunol. 1998;161:6985-92
29. Sahin U, Türeci O, Schmitt H, Cochlovius B, Johannes T, Schmits R. et al. Human neoplasms elicit multiple specific immune responses in the autologous host. Proc Natl Acad Sci U S A. 1995;92:11810-3
30. Yasuda M, Takenoyama M, Obata Y, Sugaya M, So T, Hanagiri T. et al. Tumor-infiltrating B lymphocytes as a potential source of identifying tumor antigen in human lung cancer. Cancer Res. 2002;62:1751-6
31. Sharkey MS, Lizée G, Gonzales MI, Patel S, Topalian SL. CD4(+) T-cell recognition of mutated B-RAF in melanoma patients harboring the V599E mutation. Cancer Res. 2004;64:1595-9
32. Renkvist N, Castelli C, Robbins PF, Parmiani G. A listing of human tumor antigens recognized by T cells. Cancer Immunol Immunother. 2001;50:3-15
33. Novellino L, Castelli C, Parmiani G. A listing of human tumor antigens recognized by T cells: March 2004 update. Cancer Immunol Immunother. 2005;54:187-207
34. Kawakami Y. [Identification of human tumor antigens recognized by T cells]. Nihon Rinsho. 2005;63(Suppl 4):562-7
35. Zhong S, Malecek K, Johnson LA, Yu Z, Vega-Saenz de Miera E, Darvishian F. et al. T-cell receptor affinity and avidity defines antitumor response and autoimmunity in T-cell immunotherapy. Proc Natl Acad Sci U S A. 2013;110:6973-8
36. Stone JD, Harris DT, Kranz DM. TCR affinity for p/MHC formed by tumor antigens that are self-proteins: impact on efficacy and toxicity. Curr Opin Immunol. 2015;33:16-22
37. Tomita M, Tsumoto K. Hybridoma technologies for antibody production. Immunotherapy. 2011;3:371-80
38. Zhang C. Hybridoma technology for the generation of monoclonal antibodies. Methods Mol Biol. 2012;901:117-35
39. Arimilli S, Cardoso C, Mukku P, Baichwal V, Nag B. Refolding and reconstitution of functionally active complexes of human leukocyte antigen DR2 and myelin basic protein peptide from recombinant alpha and beta polypeptide chains. J Biol Chem. 1995;270:971-7
40. Garboczi DN, Hung DT, Wiley DC. HLA-A2-peptide complexes: refolding and crystallization of molecules expressed in Escherichia coli and complexed with single antigenic peptides. Proc Natl Acad Sci U S A. 1992;89:3429-33
41. Piao WH, Song XG, Liu MC, He Y, Zhang HH, Xu WX. et al. Cloning, expression, and purification of HLA-A2-BSP and beta-2m in Escherichia coli. Protein Expr Purif. 2004;35:210-7
42. Denkberg G, Cohen CJ, Segal D, Kirkin AF, Reiter Y. Recombinant human single-chain MHC-peptide complexes made from E. coli by in vitro refolding: functional single-chain MHC-peptide complexes and tetramers with tumor associated antigens. Eur J Immunol. 2000;30:3522-32
43. Rudolf D, Silberzahn T, Walter S, Maurer D, Engelhard J, Wernet D. et al. Potent costimulation of human CD8 T cells by anti-4-1BB and anti-CD28 on synthetic artificial antigen presenting cells. Cancer Immunol Immunother. 2008;57:175-83
44. Wetzel SA, Parker DC. MHC transfer from APC to T cells following antigen recognition. Crit Rev Immunol. 2006;26:1-21
45. Tamminen WL, Wraith D, Barber BH. Searching for MHC-restricted anti-viral antibodies: antibodies recognizing the nucleoprotein of influenza virus dominate the serological response of C57BL/6 mice to syngeneic influenza-infected cells. Eur J Immunol. 1987;17:999-1006
46. Rubin B, Malissen B, Jørgensen PN, Zeuthen J. Recognition of insulin on MHC-class-II-expressing L929 cells by antibody and T cells. Res Immunol. 1989;140:67-74
47. Porgador A, Yewdell JW, Deng Y, Bennink JR, Germain RN. Localization, quantitation, and in situ detection of specific peptide-MHC class I complexes using a monoclonal antibody. Immunity. 1997;6:715-26
48. Dadaglio G, Nelson CA, Deck MB, Petzold SJ, Unanue ER. Characterization and quantitation of peptide-MHC complexes produced from hen egg lysozyme using a monoclonal antibody. Immunity. 1997;6:727-38
49. Santich BH, Liu H, Liu C, Cheung NK. Generation of TCR-Like antibodies using phage display. Methods Mol Biol. 2015;1348:191-204
50. Dahan R, Reiter Y. T-cell-receptor-like antibodies-generation, function and applications. Expert Rev Mol Med. 2012;14:e6
51. He K, Zhang X, Wang L, Du X, Wei D. Production of a soluble single-chain variable fragment antibody against okadaic acid and exploration of its specific binding. Anal Biochem. 2016;503:21-7
52. Tamminen WL, Wraith D, Barber BH. Searching for MHC-restricted anti-viral antibodies: antibodies recognizing the nucleoprotein of influenza virus dominate the serological response of C57BL/6 mice to syngeneic influenza-infected cells. Eur J Immunol. 1987;17:999-1006
53. Weiner LM. Fully human therapeutic monoclonal antibodies. J Immunother. 2006;29:1-9
54. Smith SA, Crowe JE Jr. Use of human hybridoma technology to isolate human monoclonal antibodies. Microbiol Spectr. 2015;3:Aid-0027 -2014
55. Glukhova XA, Prusakova OV, Trizna JA, Zaripov MM, Afanas'eva GV, Glukhov AS. et al. Updates on the production of therapeutic antibodies using human hybridoma technique. Curr Pharm Des. 2016;22:870-8
56. Andersen PS, Stryhn A, Hansen BE, Fugger L, Engberg J, Buus S. A recombinant antibody with the antigen-specific, major histocompatibility complex-restricted specificity of T cells. Proc Natl Acad Sci U S A. 1996;93:1820-4
57. de Haard HJ, van Neer N, Reurs A, Hufton SE, Roovers RC, Henderikx P. et al. A large non-immunized human Fab fragment phage library that permits rapid isolation and kinetic analysis of high affinity antibodies. J Biol Chem. 1999;274:18218-30
58. Frenzel A, Kügler J, Wilke S, Schirrmann T, Hust M. Construction of human antibody gene libraries and selection of antibodies by phage display. Methods Mol Biol. 2014;1060:215-43
59. Shahsavarian MA, Le Minoux D, Matti KM, Kaveri S, Lacroix-Desmazes S, Boquet D. et al. Exploitation of rolling circle amplification for the construction of large phage-display antibody libraries. J Immunol Methods. 2014;407:26-34
60. Omar N, Lim TS. Construction of naive and immune human Fab phage-display library.Methods Mol Biol. 2018. 1701:25-44
61. Chames P, Hufton SE, Coulie PG, Uchanska-Ziegler B, Hoogenboom HR. Direct selection of a human antibody fragment directed against the tumor T-cell epitope HLA-A1-MAGE-A1 from a nonimmunized phage-Fab library. Proc Natl Acad Sci U S A. 2000;97:7969-74
62. Stewart-Jones G, Wadle A, Hombach A, Shenderov E, Held G, Fischer E. et al. Rational development of high-affinity T-cell receptor-like antibodies. Proc Natl Acad Sci U S A. 2009;106:5784-8
63. Denkberg G, Lev A, Eisenbach L, Benhar I, Reiter Y. Selective targeting of melanoma and APCs using a recombinant antibody with TCR-like specificity directed toward a melanoma differentiation antigen. J Immunol. 2003;171:2197-207
64. Almagro JC, Daniels-Wells TR, Perez-Tapia SM, Penichet ML. Progress and challenges in the design and clinical development of antibodies for cancer therapy. Front Immunol. 2018;8:1751
65. Chan CE, Lim AP, MacAry PA, Hanson BJ. The role of phage display in therapeutic antibody discovery. Int Immunol. 2014;26:649-57
66. Hammers CM, Stanley JR. Antibody phage display: technique and applications. J Invest Dermatol. 2014;134:1-5
67. Frenzel A, Schirrmann T, Hust M. Phage display-derived human antibodies in clinical development and therapy. MAbs. 2016;8:1177-94
68. Dasch JR, Dasch AL. Using phage display to create recombinant antibodies. Cold Spring Harb Protoc. 2017;2017:pdb.prot093864
69. Trenevska I, Li D, Banham AH. Therapeutic antibodies against intracellular tumor antigens. Front Immunol. 2017;8:1001
70. Bernardeau K, Gouard S, David G, Ruellan AL, Devys A, Barbet J. et al. Assessment of CD8 involvement in T cell clone avidity by direct measurement of HLA-A2/Mage3 complex density using a high-affinity TCR like monoclonal antibody. Eur J Immunol. 2005;35:2864-75
71. Dubrovsky L, Dao T, Gejman RS, Brea EJ, Chang AY, Oh CY. et al. T cell receptor mimic antibodies for cancer therapy. Oncoimmunology. 2016;5:e1049803
72. Massilamany C, Krishnan B, Reddy J. Major histocompatibility complex class II dextramers:new tools for the detection of antigen-specific, CD4 T Cells in basic and clinical research. Scand J Immunol. 2015;82:399-408
73. Dolton G, Tungatt K, Lloyd A, Bianchi V, Theaker SM, Trimby A. et al. More tricks with tetramers: a practical guide to staining T cells with peptide-MHC multimers. Immunology. 2015;146:11-22
74. Izquierdo C, Ortiz AZ, Presa M, Malo S, Montoya A, Garabatos N. et al. Treatment of T1D via optimized expansion of antigen-specific Tregs induced by IL-2/anti-IL-2 monoclonal antibody complexes and peptide/MHC tetramers. Sci Rep. 2018;8:8106
75. Bedouelle H. Principles and equations for measuring and interpreting protein stability:from monomer to tetramer. Biochimie. 2016;121:29-37
76. Polakova K, Plaksin D, Chung DH, Belyakov IM, Berzofsky JA, Margulies DH. Antibodies directed against the MHC-I molecule H-2Dd complexed with an antigenic peptide: similarities to a T cell receptor with the same specificity. J Immunol. 2000;165:5703-12
77. Herschhorn A, Marasco WA, Hizi A. Antibodies and lentiviruses that specifically recognize a T cell epitope derived from HIV-1 Nef protein and presented by HLA-C. J Immunol. 2010;185:7623-32
78. Weidanz JA, Piazza P, Hickman-Miller H, Woodburn D, Nguyen T, Wahl A. et al. Development and implementation of a direct detection, quantitation and validation system for class I MHC self-peptide epitopes. J Immunol Methods. 2007;318:47-58
79. Nunoya J, Nakashima T, Kawana-Tachikawa A, Kiyotani K, Ito Y, Sugimura K. et al. Short communication: generation of recombinant monoclonal antibodies against an immunodominant HLA-A*2402-restricted HIV type 1 CTL epitope. AIDS Res Hum Retroviruses. 2009;25:897-904
80. Biddison WE, Turner RV, Gagnon SJ, Lev A, Cohen CJ, Reiter Y. Tax and M1 peptide/HLA-A2-specific Fabs and T cell receptors recognize nonidentical structural features on peptide/HLA-A2 complexes. J Immunol. 2003;171:3064-74
81. Sastry KS, Too CT, Kaur K, Gehring AJ, Low L, Javiad A. et al. Targeting hepatitis B virus-infected cells with a T-cell receptor-like antibody. J Virol. 2011;85:1935-42
82. Makler O, Oved K, Netzer N, Wolf D, Reiter Y. Direct visualization of the dynamics of antigen presentation in human cells infected with cytomegalovirus revealed by antibodies mimicking TCR specificity. Eur J Immunol. 2010;40:1552-65
83. Duc HT, Rucay P, Righenzi S, Halle-Pannenko O, Kourilsky P. Monoclonal antibodies directed against T cell epitopes presented by class I MHC antigens. Int Immunol. 1993;5:427-31
84. Wittman VP, Woodburn D, Nguyen T, Neethling FA, Wright S, Weidanz JA. Antibody targeting to a class I MHC-peptide epitope promotes tumor cell death. J Immunol. 2006;177:4187-95
85. Weidanz JA, Nguyen T, Woodburn T, Neethling FA, Chiriva-Internati M, Hildebrand WH. et al. Levels of specific peptide-HLA class I complex predicts tumor cell susceptibility to CTL killing. J Immunol. 2006;177:5088-97
86. Neethling FA, Ramakrishna V, Keler T, Buchli R, Woodburn T, Weidanz JA. Assessing vaccine potency using TCRmimic antibodies. Vaccine. 2008;26:3092-102
87. Verma B, Hawkins OE, Neethling FA, Caseltine SL, Largo SR, Hildebrand WH. et al. Direct discovery and validation of a peptide/MHC epitope expressed in primary human breast cancer cells using a TCRm monoclonal antibody with profound antitumor properties. Cancer Immunol Immunother. 2010;59:563-73
88. Hawkins O, Verma B, Lightfoot S, Jain R, Rawat A, McNair S. et al. An HLA-presented fragment of macrophage migration inhibitory factor is a therapeutic target for invasive breast cancer. J Immunol. 2011;186:6607-16
89. Li D, Bentley C, Anderson A, Wiblin S, Cleary KLS, Koustoulidou S. et al. Development of a T-cell receptor mimic antibody against wild-type p53 for cancer immunotherapy. Cancer Res. 2017;77:2699-711
90. Li D, Bentley C, Yates J, Salimi M, Greig J, Wiblin S. et al. Engineering chimeric human and mouse major histocompatibility complex (MHC) class I tetramers for the production of T-cell receptor (TCR) mimic antibodies. PLoS One. 2017;12:e0176642
91. Sergeeva A, Alatrash G, He H, Ruisaard K, Lu S, Wygant J. et al. An anti-PR1/HLA-A2 T-cell receptor-like antibody mediates complement-dependent cytotoxicity against acute myeloid leukemia progenitor cells. Blood. 2011;117:4262-72
92. Alatrash G, Mittendorf EA, Sergeeva A, Sukhumalchandra P, Qiao N, Zhang M. et al. Broad cross-presentation of the hematopoietically derived PR1 antigen on solid tumors leads to susceptibility to PR1-targeted immunotherapy. J Immunol. 2012;189:5476-84
93. Dao T, Yan S, Veomett N, Pankov D, Zhou L, Korontsvit T. et al. Targeting the intracellular WT1 oncogene product with a therapeutic human antibody. Sci Transl Med. 2013;5:176ra33
94. Veomett N, Dao T, Liu H, Xiang J, Pankov D, Dubrovsky L. et al. Therapeutic efficacy of an Fc-enhanced TCR-like antibody to the intracellular WT1 oncoprotein. Clin Cancer Res. 2014;20:4036-46
95. Chames P, Willemsen RA, Rojas G, Dieckmann D, Rem L, Schuler G. et al. TCR-like human antibodies expressed on human CTLs mediate antibody affinity-dependent cytolytic activity. J Immunol. 2002;169:1110-8
96. Denkberg G, Klechevsky E, Reiter Y. Modification of a tumor-derived peptide at an HLA-A2 anchor residue can alter the conformation of the MHC-peptide complex: probing with TCR-like recombinant antibodies. J Immunol. 2002;169:4399-407
97. Lev A, Denkberg G, Cohen CJ, Tzukerman M, Skorecki KL, Chames P. et al. Isolation and characterization of human recombinant antibodies endowed with the antigen-specific, major histocompatibility complex-restricted specificity of T cells directed toward the widely expressed tumor T-cell epitopes of the telomerase catalytic subunit. Cancer Res. 2002;62:3184-94
98. Held G, Matsuo M, Epel M, Gnjatic S, Ritter G, Lee SY. et al. Dissecting cytotoxic T cell responses towards the NY-ESO-1 protein by peptide/MHC-specific antibody fragments. Eur J Immunol. 2004;34:2919-29
99. Held G, Wadle A, Dauth N, Stewart-Jones G, Sturm C, Thiel M. et al. MHC-peptide-specific antibodies reveal inefficient presentation of an HLA-A*0201-restricted, Melan-A-derived peptide after active intracellular processing. Eur J Immunol. 2007;37:2008-17
100. Pastan I, Kreitman RJ. Immunotoxins in cancer therapy. Curr Opin Investig Drugs. 2002;3:1089-91
101. Cohen CJ, Hoffmann N, Farago M, Hoogenboom HR, Eisenbach L, Reiter Y. Direct detection and quantitation of a distinct T-cell epitope derived from tumor-specific epithelial cell-associated mucin using human recombinant antibodies endowed with the antigen-specific, major histocompatibility complex-restricted specificity of T cells. Cancer Res. 2002;62:5835-44
102. Klechevsky E, Gallegos M, Denkberg G, Palucka K, Banchereau J, Cohen C. et al. Antitumor activity of immunotoxins with T-cell receptor-like specificity against human melanoma xenografts. Cancer Res. 2008;68:6360-7
103. Epel M, Carmi I, Soueid-Baumgarten S, Oh S, Bera T, Pastan I. et al. Targeting TARP, a novel breast and prostate tumor-associated antigen, with T cell receptor-like human recombinant antibodies. Eur J Immunol. 2008;38:1706-20
104. Michaeli Y, Denkberg G, Sinik K, Lantzy L, Chih-Sheng C, Beauverd C. et al. Expression hierarchy of T cell epitopes from melanoma differentiation antigens: unexpected high level presentation of tyrosinase-HLA-A2 complexes revealed by peptide-specific, MHC-restricted, TCR-like antibodies. J Immunol. 2009;182:6328-41
105. Rafiq S, Purdon TJ, Daniyan AF, Koneru M, Dao T, Liu C. et al. Optimized T-cell receptor-mimic chimeric antigen receptor T cells directed toward the intracellular Wilms tumor 1 antigen. Leukemia. 2017;31:1788-97
106. Sugiyama H. WT1 (Wilms' tumor gene 1): biology and cancer immunotherapy. Jpn J Clin Oncol. 2010;40:377-87
107. Di Stasi A, Jimenez AM, Minagawa K, Al-Obaidi M, Rezvani K. Review of the results of WT1 peptide vaccination strategies for myelodysplastic syndromes and acute myeloid leukemia from nine different studies. Front Immunol. 2015;6:36
108. Morrison AA, Viney RL, Ladomery MR. The post-transcriptional roles of WT1, a multifunctional zinc-finger protein. Biochim Biophys Acta. 2008;1785:55-62
109. Liu H, Xu Y, Xiang J, Long L, Green S, Yang Z. et al. Targeting alpha-fetoprotein (AFP)-MHC complex with CAR T-cell therapy for liver cancer. Clin Cancer Res. 2017;23:478-88
110. Bei R, Mizejewski GJ. Alpha fetoprotein is more than a hepatocellular cancer biomarker: from spontaneous immune response in cancer patients to the development of an AFP-based cancer vaccine. Curr Mol Med. 2011;11:564-81
111. Li M, Li H, Li C, Wang S, Jiang W, Liu Z. et al. Alpha-fetoprotein: a new member of intracellular signal molecules in regulation of the PI3K/AKT signaling in human hepatoma cell lines. Int J Cancer. 2011;128:524-32
112. Fang ZL, Fang N, Han XN, Huang G, Fu XJ, Xie GS. et al. Effects of AFP gene silencing on survivin mRNA expression inhibition in HepG2 cells. Genet Mol Res. 2015;14:3184-90
113. Ma Q, Garber HR, Lu S, He H, Tallis E, Ding X. et al. A novel TCR-like CAR with specificity for PR1/HLA-A2 effectively targets myeloid leukemia in vitro when expressed in human adult peripheral blood and cord blood T cells. Cytotherapy. 2016;18:985-94
114. Zhao Q, Ahmed M, Tassev DV, Hasan A, Kuo TY, Guo HF. et al. Affinity maturation of T-cell receptor-like antibodies for Wilms tumor 1 peptide greatly enhances therapeutic potential. Leukemia. 2015;29:2238-47
115. Oren R, Hod-Marco M, Haus-Cohen M, Thomas S, Blat D, Duvshani N. et al. Functional comparison of engineered T cells carrying a native TCR versus TCR-like antibody-based chimeric antigen receptors indicates affinity/avidity thresholds. J Immunol. 2014;193:5733-43
116. Inaguma Y, Akahori Y, Murayama Y, Shiraishi K, Tsuzuki-Iba S, Endoh A. et al. Construction and molecular characterization of a T-cell receptor-like antibody and CAR-T cells specific for minor histocompatibility antigen HA-1H. Gene Ther. 2014;21:575-84
117. Zhang G, Wang L, Cui H, Wang X, Zhang G, Ma J. et al. Anti-melanoma activity of T cells redirected with a TCR-like chimeric antigen receptor. Sci Rep. 2014;4:3571
118. Maus MV, Plotkin J, Jakka G, Stewart-Jones G, Rivière I, Merghoub T. et al. An MHC-restricted antibody-based chimeric antigen receptor requires TCR-like affinity to maintain antigen specificity. Mol Ther Oncolytics. 2017;3:1-9
119. Villamor N, Montserrat E, Colomer D. Mechanism of action and resistance to monoclonal antibody therapy. Semin Oncol. 2003;30:424-33
120. Zheng J, Guo Y, Ji X, Cui L, He W. A novel antibody-like TCRγδ-Ig fusion protein exhibits antitumoractivity against human ovarian carcinoma. Cancer Lett. 2013;341:150-8
121. Miller AS, Tejada ML, Gazzano-Santoro H. Methods for measuring antibody-dependent cell-mediated cytotoxicity in vitro. Methods Mol Biol. 2014;1134:59-65
122. Verma B, Neethling FA, Caseltine S, Fabrizio G, Largo S, Duty JA. et al. TCR mimic monoclonal antibody targets a specific peptide/HLA class I complex and significantly impedes tumor growth in vivo using breast cancer models. J Immunol. 2010;184:2156-65
123. Kerndrup G, Meyer K, Ellegaard J, Hokland P. Natural killer (NK)-cell activity and antibody-dependent cellular cytotoxicity (ADCC) in primary preleukemic syndrome. Leuk Res. 1984;8:239-47
124. Munn DH, Cheung NK. Phagocytosis of tumor cells by human monocytes cultured in recombinant macrophage colony-stimulating factor. J Exp Med. 1990;172:231-7
125. van der Bij GJ, Bögels M, Otten MA, Oosterling SJ, Kuppen PJ, Meijer S. et al. Experimentally induced liver metastases from colorectal cancer can be prevented by mononuclear phagocyte-mediated monoclonal antibody therapy. J Hepatol. 2010;53:677-85
126. Verma B, Jain R, Caseltine S, Rennels A, Bhattacharya R, Markiewski MM. et al. TCR mimic monoclonal antibodies induce apoptosis of tumor cells via immune effector-independent mechanisms. J Immunol. 2011;186:3265-76
127. Vezina HE, Cotreau M, Han TH, Gupta M. Antibody-drug conjugates as cancer therapeutics:past,present, and future. J Clin Pharmacol. 2017;57(Suppl 10):S11-S25
128. Smaglo BG, Aldeghaither D, Weiner LM. The development of immunoconjugates for targeted cancer therapy. Nat Rev Clin Oncol. 2014;11:637-48
129. Diamantis N, Banerji U. Antibody-drug conjugates-an emerging class of cancer treatment. Br J Cancer. 2016;114:362-7
130. Alewine C, Hassan R, Pastan I. Advances in anticancer immunotoxin therapy. Oncologist. 2015;20:176-85
131. Allahyari H, Heidari S, Ghamgosha M, Saffarian P, Amani J. Immunotoxin: a new tool for cancer therapy. Tumour Biol. 2017;39:1010428317692226
132. Flavell DJ. Countering immunotoxin immunogenicity. Br J Cancer. 2016;114:1177-9
133. Golubovskaya V, Wu L. Different subsets of T Cells, memory, effector functions, and CAR-T immunotherapy. Cancers (Basel). 2016:8
134. Wang Z, Wu Z, Liu Y, Han W. New development in CAR-T cell therapy. J Hematol Oncol. 2017;10:53
135. Pettitt D, Arshad Z, Smith J, Stanic T, Holländer G, Brindley D. CAR-T cells: A systematic review and mixed methods analysis of the clinical trial landscape. Mol Ther. 2018;26:342-53
136. Eshhar Z, Waks T, Bendavid A, Schindler DG. Functional expression of chimeric receptor genes in human T cells. J Immunol Methods. 2001;248:67-76
137. Stauss HJ, Cesco-Gaspere M, Thomas S, Hart DP, Xue SA, Holler A. et al. Monoclonal T-cell receptors: new reagents for cancer therapy. Mol Ther. 2007;15:1744-50
138. Dolan BP. Quantitating MHC class I ligand production and presentation using TCR-Like antibodies.Methods Mol Biol. 2019. 1988:149-57
139. Dolan BP. Quantitating MHC class I ligand production and presentation using TCR-like antibodies. Methods Mol Biol. 2013;960:169-77
140. Zehn D, Cohen CJ, Reiter Y, Walden P. Extended presentation of specific MHC-peptide complexes by mature dendritic cells compared to other types of antigen-presenting cells. Eur J Immunol. 2004;34:1551-60
141. Kugler A, Stuhler G, Walden P, Zöller G, Zobywalski A, Brossart P. et al. Regression of human metastatic renal cell carcinoma after vaccination with tumor cell-dendritic cell hybrids. Nat Med. 2000;6:332-6
142. Chen S, Li Y, Depontieu FR, McMiller TL, English AM, Shabanowitz J. et al. Structure-based design of altered MHC class II-restricted peptide ligands with heterogeneous immunogenicity. J Immunol. 2013;191:5097-106
143. Khan JM, Cheruku HR, Tong JC, Ranganathan S. MPID-T2: a database for sequence-structure-function analyses of pMHC and TR/pMHC structures. Bioinformatics. 2011;27:1192-3
144. Yu ED, Girardi E, Wang J, Mac TT, Yu KO, Van Calenbergh S. et al. Structural basis for the recognition of C20:2-αGalCer by the invariant natural killer T cell receptor-like antibody L363. J Biol Chem. 2012;287:1269-78
145. Stryhn A, Andersen PS, Pedersen LO, Svejgaard A, Holm A, Thorpe CJ. et al. Shared fine specificity between T-cell receptors and an antibody recognizing a peptide/major histocompatibility class I complex. Proc Natl Acad Sci U S A. 1996;93:10338-42
146. Rognan D, Stryhn A, Fugger L, Lyngbaek S, Engberg J, Andersen PS. et al. Modeling the interactions of a peptide-major histocompatibility class I ligand with its receptors. I. Recognition by two alpha beta T cell receptors. J Comput Aided Mol Des. 2000;14:53-69
147. Sharma AK, Kuhns JJ, Yan S, Friedline RH, Long B, Tisch R. et al. Class I major histocompatibility complex anchor substitutions alter the conformation of T cell receptor contacts. J Biol Chem. 2001;276:21443-9
148. Hülsmeyer M, Chames P, Hillig RC, Stanfield RL, Held G, Coulie PG. et al. A major histocompatibility complex-peptide-restricted antibody and T cell receptor molecules recognize their target by distinct binding modes: crystal structure of human leukocyte antigen (HLA)-A1-MAGE-A1 in complex with Fab-hyb3. J Biol Chem. 2005;280:2972-80
149. Kumar P, Vahedi-Faridi A, Saenger W, Ziegler A, Uchanska-Ziegler B. Conformational changes within the HLA-A1:MAGE-A1 complex induced by binding of a recombinant antibody fragment with TCR-like specificity. Protein Sci. 2009;18:37-49
150. Cloutier SM, Couty S, Terskikh A, Marguerat L, Crivelli V, Pugnières M. et al. Streptabody, a high avidity molecule made by tetramerization of in vivo biotinylated, phage display-selected scFv fragments on streptavidin. Mol Immunol. 2000;37:1067-77
151. FitzGerald DJ, Kreitman R, Wilson W, Squires D, Pastan I. Recombinant immunotoxins for treating cancer. Int J Med Microbiol. 2004;293:577-82
152. Lai J, Wang Y, Wu SS, Ding D, Sun ZY, Zhang Y. et al. Elimination of melanoma by sortase A-generated TCR-like antibody-drug conjugates (TL-ADCs) targeting intracellular melanoma antigen MART-1. Biomaterials. 2018;178:158-69
153. Denkberg G, Stronge VS, Zahavi E, Pittoni P, Oren R, Shepherd D. et al. Phage display-derived recombinant antibodies with TCR-like specificity against alpha-galactosylceramide and its analogues in complex with human CD1d molecules. Eur J Immunol. 2008;38:829-40
154. Lowe DB, Bivens CK, Mobley AS, Herrera CE, McCormick AL, Wichner T. et al. TCR-like antibody drug conjugates mediate killing of tumor cells with low peptide/HLA targets. MAbs. 2017;9:603-14
155. Lode HN, Reisfeld RA. Targeted cytokines for cancer immunotherapy. Immunol Res. 2000;21:279-88
156. Ji C, Sastry KS, Tiefenthaler G, Cano J, Tang T, Ho ZZ. et al. Targeted delivery of interferon-alpha to hepatitis B virus-infected cells using T-cell receptor-like antibodies. Hepatology. 2012;56:2027-38
157. Withoff S, Helfrich W, De Leij LF, Molema G. Bi-specific antibody therapy for the treatment of cancer. Curr Opin Mol Ther. 2001;3:53-62
158. Herrmann AC, Im JS, Pareek S, Ruiz-Vasquez W, Lu S, Sergeeva A. et al. A novel T-cell engaging bi-specific antibody targeting the leukemia antigen PR1/HLA-A2. Front Immunol. 2019;9:3153
159. Ahmed M, Lopez-Albaitero A, Pankov D, Santich BH, Liu H, Yan S. et al. TCR-mimic bispecific antibodies targeting LMP2A show potent activity against EBV malignancies. JCI Insight. 2018:3
160. Dao T, Pankov D, Scott A, Korontsvit T, Zakhaleva V, Xu Y. et al. Therapeutic bispecific T-cell engager antibody targeting the intracellular oncoprotein WT1. Nat Biotechnol. 2015;33:1079-86
161. Imai N, Ikeda H, Shiku H. [Targeting cancer antigen (MAGE-A4, NY-ESO-1) for immunotherapy]. Nihon Rinsho. 2012;70:2125-9
162. May RJ, Dao T, Pinilla-Ibarz J, Korontsvit T, Zakhaleva V, Zhang RH. et al. Peptide epitopes from the Wilms' tumor 1 oncoprotein stimulate CD4+ and CD8+ T cells that recognize and kill human malignant mesothelioma tumor cells. Clin Cancer Res. 2007;13:4547-55
163. Mizukoshi E, Nakamoto Y, Tsuji H, Yamashita T, Kaneko S. Identification of alpha-fetoprotein-derived peptides recognized by cytotoxic T lymphocytes in HLA-A24+ patients with hepatocellular carcinoma. Int J Cancer. 2006;118:1194-204
164. Asai H, Fujiwara H, An J, Ochi T, Miyazaki Y, Nagai K. et al. Co-introduced functional CCR2 potentiates in vivo anti-lung cancer functionality mediated by T cells double gene-modified to express WT1-specific T-cell receptor. PLoS One. 2013;8:e56820
165. Bianchi V, Bulek A, Fuller A, Lloyd A, Attaf M, Rizkallah PJ. et al. A molecular switch abrogates glycoprotein 100 (gp100) T-cell Receptor (TCR) targeting of a human melanoma antigen. J Biol Chem. 2016;291:8951-9
166. Zolghadri S, Bahrami A, Hassan Khan MT, Munoz-Munoz J, Garcia-Molina F, Garcia-Canovas F. et al. A comprehensive review on tyrosinase inhibitors. J Enzyme Inhib Med Chem. 2019;34:279-309
167. Ba S, Vinoth Kumar V. Recent developments in the use of tyrosinase and laccase in environmental applications. Crit Rev Biotechnol. 2017;37:819-32
168. Etzkorn JR, Jew OS, Shin TM, Sobanko JF, Neal DE, Miller CJ. Mohs micrographic surgery with melanoma antigen recognized by T cells 1 (MART-1) immunostaining for atypical intraepidermal melanocytic proliferation. J Am Acad Dermatol. 2018;79:1109-16.e1
169. Alatrash G, Perakis AA, Kerros C, Peters HL, Sukhumalchandra P, Zhang M. et al. Targeting the leukemia antigen PR1 with immunotherapy for the treatment of multiple myeloma. Clin Cancer Res. 2018;24:3386-96
170. Azemar M, Djahansouzi S, Jäger E, Solbach C, Schmidt M, Maurer AB. et al. Regression of cutaneous tumor lesions in patients intratumorally injected with a recombinant single-chain antibody-toxin targeted to ErbB2/HER2. Breast Cancer Res Treat. 2003;82:155-64
171. Lau JL, Fowler JE Jr, Ghosh L. Epidermal growth factor in the normal and neoplastic kidney and bladder. J Urol. 1988;139:170-5
172. Groeben C, Wirth MP. Prostate cancer: basics on clinical appearance, diagnostics and treatment. Med Monatsschr Pharm. 2017;40:192-201
173. Barriuso Feijóo J, Sundlov A, González Barón M. [Antibodies against cancer]. Rev Clin Esp. 2004;204:649-54
174. Mohammadzadeh M, Shirmohammadi M, Ghojazadeh M, Nikniaz L, Raeisi M, Aghdas SAM. Dendritic cells pulsed with prostate-specific membrane antigen in metastatic castration-resistant prostate cancer patients: a systematic review and meta-analysis. Prostate Int. 2018;6:119-25
175. Modjtahedi H, Moscatello DK, Box G, Green M, Shotton C, Lamb DJ. et al. Targeting of cells expressing wild-type EGFR and type-III mutant EGFR (EGFRvIII) by anti-EGFR MAb ICR62: a two-pronged attack for tumour therapy. Int J Cancer. 2003;105:273-80
176. Jurišić V, Obradovic J, Pavlović S, Djordjevic N. Epidermal growth factor receptor gene in non-small-cell lung cancer:the importance of promoter polymorphism investigation. Anal Cell Pathol (Amst). 2018;2018:6192187
177. Worley BS, van den Broeke LT, Goletz TJ, Pendleton CD, Daschbach EM, Thomas EK. et al. Antigenicity of fusion proteins from sarcoma-associated chromosomal translocations. Cancer Res. 2001;61:6868-75
178. Errington JA, Conway RM, Walsh-Conway N, Browning J, Freyer C, Cebon J. et al. Expression of cancer-testis antigens (MAGE-A1, MAGE-A3/6, MAGE-A4, MAGE-C1 and NY-ESO-1) in primary human uveal and conjunctival melanoma. Br J Ophthalmol. 2012;96:451-8
179. Disis ML, Cheever MA. HER-2/neu protein: a target for antigen-specific immunotherapy of human cancer. Adv Cancer Res. 1997;71:343-71
180. Boël P, Wildmann C, Sensi ML, Brasseur R, Renauld JC, Coulie P. et al. BAGE: a new gene encoding an antigen recognized on human melanomas by cytolytic T lymphocytes. Immunity. 1995;2:167-75
181. Gaugler B, Van den Eynde B, van der Bruggen P, Romero P, Gaforio JJ, De Plaen E. et al. Human gene MAGE-3 codes for an antigen recognized on a melanoma by autologous cytolytic T lymphocytes. J Exp Med. 1994;179:921-30
182. Kawakami Y, Eliyahu S, Sakaguchi K, Robbins PF, Rivoltini L, Yannelli JR. et al. Identification of the immunodominant peptides of the MART-1 human melanoma antigen recognized by the majority of HLA-A2-restricted tumor infiltrating lymphocytes. J Exp Med. 1994;180:347-52
183. Wang RF, Robbins PF, Kawakami Y, Kang XQ, Rosenberg SA. Identification of a gene encoding a melanoma tumor antigen recognized by HLA-A31-restricted tumor-infiltrating lymphocytes. J Exp Med. 1995;181:799-804
184. Oka Y, Elisseeva OA, Tsuboi A, Ogawa H, Tamaki H, Li H. et al. Human cytotoxic T-lymphocyte responses specific for peptides of the wild-type Wilms' tumor gene (WT1) product. Immunogenetics. 2000;51:99-107
185. Wang RF, Appella E, Kawakami Y, Kang X, Rosenberg SA. Identification of TRP-2 as a human tumor antigen recognized by cytotoxic T lymphocytes. J Exp Med. 1996;184:2207-16
186. Anichini A, Maccalli C, Mortarini R, Salvi S, Mazzocchi A, Squarcina P. et al. Melanoma cells and normal melanocytes share antigens recognized by HLA-A2-restricted cytotoxic T cell clones from melanoma patients. J Exp Med. 1993;177:989-98
187. Coulie PG, Brichard V, Van Pel A, Wölfel T, Schneider J, Traversari C. et al. A new gene coding for a differentiation antigen recognized by autologous cytolytic T lymphocytes on HLA-A2 melanomas. J Exp Med. 1994;180:35-42
188. Celis E, Tsai V, Crimi C, DeMars R, Wentworth PA, Chesnut RW. et al. Induction of anti-tumor cytotoxic T lymphocytes in normal humans using primary cultures and synthetic peptide epitopes. Proc Natl Acad Sci U S A. 1994;91:2105-9
189. Castelli C, Storkus WJ, Maeurer MJ, Martin DM, Huang EC, Pramanik BN. et al. Mass spectrometric identification of a naturally processed melanoma peptide recognized by CD8+ cytotoxic T lymphocytes. J Exp Med. 1995;181:363-8
190. Luckey CJ, King GM, Marto JA, Venketeswaran S, Maier BF, Crotzer VL. et al. Proteasomes can either generate or destroy MHC class I epitopes: evidence for nonproteasomal epitope generation in the cytosol. J Immunol. 1998;161:112-21
191. Weinstein JN, Collisson EA, Mills GB, Shaw KR, Ozenberger BA, Ellrott K. et al. The cancer genome Atlas pan-cancer analysis project. Nat Genet. 2013;45:1113-20
192. Janahi EM, Dhasmana A, Srivastava V, Sarangi AN, Raza S, Arif JM. et al. In silico CD4+, CD8+ T-cell and B-cell immunity associated immunogenic epitope prediction and HLA distribution analysis of Zika virus. EXCLI J. 2017;16:63-72
193. Kawakami Y, Fujita T, Matsuzaki Y, Sakurai T, Tsukamoto M, Toda M. et al. Identification of human tumor antigens and its implications for diagnosis and treatment of cancer. Cancer Sci. 2004;95:784-91
194. Chang AY, Gejman RS, Brea EJ, Oh CY, Mathias MD, Pankov D. et al. Opportunities and challenges for TCR mimic antibodies in cancer therapy. Expert Opin Biol Ther. 2016;16:979-87
195. Dunbar J, Knapp B, Fuchs A, Shi J, Deane CM. Examining variable domain orientations in antigen receptors gives insight into TCR-like antibody design. PLoS Comput Biol. 2014;10:e1003852
196. Sullivan MA, Brooks LR, Weidenborner P, Domm W, Mattiacio J, Xu Q. et al. Anti-idiotypic monobodies derived from a fibronectin scaffold. Biochemistry. 2013;52:1802-13
197. Chang AY, Dao T, Gejman RS, Jarvis CA, Scott A, Dubrovsky L. et al. A therapeutic T cell receptor mimic antibody targets tumor-associated PRAME peptide/HLA-I antigens. J Clin Invest. 2017;127:3557
198. Sergeeva A, He H, Ruisaard K, St John L, Alatrash G, Clise-Dwyer K. et al. Activity of 8F4, a T-cell receptor-like anti-PR1/HLA-A2 antibody, against primary human AML in vivo. Leukemia. 2016;30:1475-84
199. Schrump DS, Fischette MR, Nguyen DM, Zhao M, Li X, Kunst TF. et al. Phase I study of decitabine-mediated gene expression in patients with cancers involving the lungs, esophagus, or pleura. Clin Cancer Res. 2006;12:5777-85
200. Gang AO, Frøsig TM, Brimnes MK, Lyngaa R, Treppendahl MB, Grønbæk K. et al. 5-Azacytidine treatment sensitizes tumor cells to T-cell mediated cytotoxicity and modulates NK cells in patients with myeloid malignancies. Blood Cancer J. 2014;4:e197
201. Bassani-Sternberg M, Pletscher-Frankild S, Jensen LJ, Mann M. Mass spectrometry of human leukocyte antigen class I peptidomes reveals strong effects of protein abundance and turnover on antigen presentation. Mol Cell Proteomics. 2015;14:658-73
202. Enenkel C. Proteasome dynamics.Biochim Biophys Acta. 2014. 1843:39-46
203. Chen Y, Zhang Y, Guo X. Proteasome dysregulation in human cancer: implications for clinical therapies. Cancer Metastasis Rev. 2017;36:703-16
204. Ma W, Vigneron N, Chapiro J, Stroobant V, Germeau C, Boon T. et al. A MAGE-C2 antigenic peptide processed by the immunoproteasome is recognized by cytolytic T cells isolated from a melanoma patient after successful immunotherapy. Int J Cancer. 2011;129:2427-34
205. Valmori D, Gileadi U, Servis C, Dunbar PR, Cerottini JC, Romero P. et al. Modulation of proteasomal activity required for the generation of a cytotoxic T lymphocyte-defined peptide derived from the tumor antigen MAGE-3. J Exp Med. 1999;189:895-906
206. Cabrera T, Angustias Fernandez M, Sierra A, Garrido A, Herruzo A, Escobedo A. et al. High frequency of altered HLA class I phenotypes in invasive breast carcinomas. Hum Immunol. 1996;50:127-34
207. Mimura K, Shiraishi K, Mueller A, Izawa S, Kua LF, So J. et al. The MAPK pathway is a predominant regulator of HLA-A expression in esophageal and gastric cancer. J Immunol. 2013;191:6261-72
208. Pollack BP, Sapkota B, Cartee TV. Epidermal growth factor receptor inhibition augments the expression of MHC class I and II genes. Clin Cancer Res. 2011;17:4400-13
209. Palmisano GL, Pistillo MP, Capanni P, Pera C, Nicolò G, Salvi S. et al. Investigation of HLA class I downregulation in breast cancer by RT-PCR. Hum Immunol. 2001;62:133-9
210. Krogsgaard M, Wucherpfennig KW, Cannella B, Hansen BE, Svejgaard A, Pyrdol J. et al. Visualization of myelin basic protein (MBP) T cell epitopes in multiple sclerosis lesions using a monoclonal antibody specific for the human histocompatibility leukocyte antigen (HLA)-DR2-MBP 85-99 complex. J Exp Med. 2000;191:1395-412
211. Dustin ML. T-cell activation through immunological synapses and kinapses. Immunol Rev. 2008;221:77-89
212. Aharoni R, Teitelbaum D, Arnon R, Puri J. Immunomodulation of experimental allergic encephalomyelitis by antibodies to the antigen-Ia complex. Nature. 1991;351:147-50
213. Uchańska-Ziegler B, Nössner E, Schenk A, Ziegler A, Schendel DJ. Soluble T cell receptor-like properties of an HLA-B35-specific monoclonal antibody (TU165). Eur J Immunol. 1993;23:734-8
214. Abastado JP, Darche S, Jouin H, Delarbre C, Gachelin G, Kourilsky P. A monoclonal antibody recognizes a subset of the H-2Dd mouse major class I antigens. Res Immunol. 1989;140:581-94
215. Garcia KC, Teyton L, Wilson IA. Structural basis of T cell recognition. Annu Rev Immunol. 1999;17:369-97
216. Lehner PJ, Cresswell P. Recent developments in MHC-class-I-mediated antigen presentation. Curr Opin Immunol. 2004;16:82-9
217. Linette GP, Stadtmauer EA, Maus MV, Rapoport AP, Levine BL, Emery L. et al. Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood. 2013;122:863-71
218. González-Galarza FF, Takeshita LY, Santos EJ, Kempson F, Maia MH, da Silva AL. et al. Allele frequency net 2015 update: new features for HLA epitopes, KIR and disease and HLA adverse drug reaction associations. Nucleic Acids Res. 2015;43:D784-8
219. Ellis JM, Henson V, Slack R, Ng J, Hartzman RJ, Katovich Hurley C.Frequencies of HLA-A2 alleles in five U.S. population groups.Predominance of A*02011 and identification of HLA-A*0231. Hum Immunol. 2000;61:334-40
220. Cao K, Hollenbach J, Shi X, Shi W, Chopek M, Fernández-Viña MA. Analysis of the frequencies of HLA-A, B, and C alleles and haplotypes in the five major ethnic groups of the United States reveals high levels of diversity in these loci and contrasting distribution patterns in these populations. Hum Immunol. 2001;62:1009-30
221. Nunes JM, Buhler S, Roessli D, Sanchez-Mazas A. The HLA-net GENE[RATE] pipeline for effective HLA data analysis and its application to 145 population samples from Europe and neighbouring areas. Tissue antigens. 2014;83:307-23
222. Nakamoto Y, Kaneko S, Takizawa H, Kikumoto Y, Takano M, Himeda Y. et al. Analysis of the CD8-positive T cell response in Japanese patients with chronic hepatitis C using HLA-A*2402 peptide tetramers. J Med Virol. 2003;70:51-61
223. Huehls AM, Coupet TA, Sentman CL. Bispecific T-cell engagers for cancer immunotherapy. Immunol Cell Biol. 2015;93:290-6
224. Curran KJ, Pegram HJ, Brentjens RJ. Chimeric antigen receptors for T cell immunotherapy: current understanding and future directions. J Gene Med. 2012;14:405-15
225. Srivastava S, Riddell SR. Engineering CAR-T cells: design concepts. Trends Immunol. 2015;36:494-502
226. van der Stegen SJ, Hamieh M, Sadelain M. The pharmacology of second-generation chimeric antigen receptors. Nat Rev Drug Discov. 2015;14:499-509
227. Perica K, Curran KJ, Brentjens RJ, Giralt SA. Building a CAR garage: preparing for the delivery of commercial CAR T cell products at memorial sloan kettering cancer center. Biol Blood Marrow Transplant. 2018;24:1135-41
228. Cummins KD, Gill S. Anti-CD123 chimeric antigen receptor T-cells (CART): an evolving treatment strategy for hematological malignancies, and a potential ace-in-the-hole against antigen-negative relapse. Leuk Lymphoma. 2018;59:1539-53
229. Fraietta JA, Nobles CL, Sammons MA, Lundh S, Carty SA, Reich TJ. et al. Disruption of TET2 promotes the therapeutic efficacy of CD19-targeted T cells. Nature. 2018;558:307-12
230. Tanaka T, Lobato MN, Rabbitts TH. Single domain intracellular antibodies: a minimal fragment for direct in vivo selection of antigen-specific intrabodies. J Mol Biol. 2003;331:1109-20
231. Streltsov V, Nuttall S. Do sharks have a new antibody lineage? Immunol Lett. 2005;97:159-60
232. Skottrup PD. Structural insights into a high affinity nanobody: antigen complex by homology modelling. J Mol Graph Model. 2017;76:305-12
233. Fan K, Jiang B, Guan Z, He J, Yang D, Xie N. et al. Fenobody: a ferritin-displayed nanobody with high apparent affinity and half-life extension. Anal Chem. 2018;90:5671-7
234. Khodabakhsh F, Behdani M, Rami A, Kazemi-Lomedasht F. Single-domain antibodies or nanobodies: a class of next-generation antibodies. Int Rev Immunol. 2018;37:316-22
235. Hajari Taheri F, Hassani M, Sharifzadeh Z, Behdani M, Arashkia A, Abolhassani M. T cell engineered with a novel nanobody-based chimeric antigen receptor against VEGFR2 as a candidate for tumor immunotherapy. IUBMB life. 2019;71:1259-1267
236. Hassani M, Hajari Taheri F, Sharifzadeh Z, Arashkia A, Hadjati J, van Weerden WM. et al. Construction of a chimeric antigen receptor bearing a nanobody against prostate a specific membrane antigen in prostate cancer. J Cell Biochem. 2019;120:10787-95
237. Xie YJ, Dougan M, Jailkhani N, Ingram J, Fang T, Kummer L. et al. Nanobody-based CAR T cells that target the tumor microenvironment inhibit the growth of solid tumors in immunocompetent mice. Proc Natl Acad Sci U S A. 2019;116:7624-31
238. Zhang G, Liu R, Zhu X, Wang L, Ma J, Han H. et al. Retargeting NK-92 for anti-melanoma activity by a TCR-like single-domain antibody. Immunol Cell Biol. 2013;91:615-24
Author contact
![]() Corresponding author: Professor Xiaoling Lu, Ph.D., Nanobody Research Center, Guangxi Medical University, Nanning, Guangxi, 530021, China, Tel: (86) 771-2387 518; Fax: (86) 771-2387 518; E-mail: luxiaolingedu.cn.
Corresponding author: Professor Xiaoling Lu, Ph.D., Nanobody Research Center, Guangxi Medical University, Nanning, Guangxi, 530021, China, Tel: (86) 771-2387 518; Fax: (86) 771-2387 518; E-mail: luxiaolingedu.cn.
 Global reach, higher impact
Global reach, higher impact