13.3
Impact Factor
Theranostics 2019; 9(25):7730-7748. doi:10.7150/thno.37306 This issue Cite
Review
The yin and yang of imaging tumor associated macrophages with PET and MRI
1. Department of Bioengineering, Rice University, Houston, TX-77054, United States
2. Werner Siemens Imaging Center, Department of Preclinical Imaging and Radiopharmacy, University Hospital Tuebingen, Eberhard Karls University, Tuebingen, Germany
3. Department of Internal Medicine VIII, University Hospital Tuebingen, Eberhard Karls University, Tuebingen, Germany
4. Department of Radiology, Stanford University, 725 Welch Rd Stanford, CA 94305-5614
‡Equal contribution
*Equal contribution
Received 2019-6-4; Accepted 2019-8-27; Published 2019-10-15
Abstract
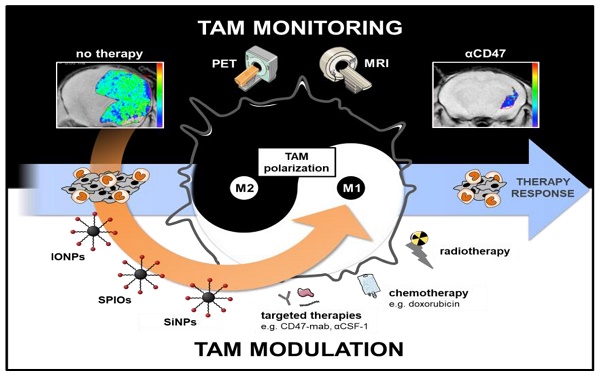
Tumor associated macrophages (TAM) are key players in the cancer microenvironment. Molecular imaging modalities such as MRI and PET can be used to track and monitor TAM dynamics in tumors non-invasively, based on specific uptake and quantification of MRI-detectable nanoparticles or PET-detectable radiotracers. Particular molecular signatures can be leveraged to target anti-inflammatory TAM, which support tumor growth, and pro-inflammatory TAM, which suppress tumor growth. In addition, TAM-directed imaging probes can be designed to include immune modulating properties, thereby leading to combined diagnostic and therapeutic (theranostic) effects. In this review, we will discuss the complementary role of TAM-directed radiotracers and iron oxide nanoparticles for monitoring cancer immunotherapies with PET and MRI technologies. In addition, we will outline how TAM-directed imaging and therapy is interdependent and can be connected towards improved clinical outcomes
Keywords: MRI, PET, Cancer Immunotherapy, Nanoparticles, Radiotracers, Immunotheranostics, Macrophages
Introduction
The immune response to cancer follows two opposite and apparently contradictory principles: some aspects of the tumor immune response can inhibit tumor growth, while others can promote tumor growth [1, 2]. Preclinical and clinical evidence showed that an overbearing pro-tumorigenic immune response in malignant tumors significantly promotes tumor growth and metastasis [1, 2]. In a pro-tumoral microenvironment, inflammatory M2 tumor associated macrophages (TAM) represent up to 50% of the tumor cell mass [3-6] while immune protective M1 TAM phenotypes are sparse [6-9]. Thus, in malignant tumors, the bulk of macrophages in the tumor tissue promote tumor growth. M2 phenotypes augment tumor cell proliferation via elaboration of cytokines, chemokines, proteases and reactive oxygen species [10-12]. In addition, M2 TAM enhance angiogenesis via regulating VEGF bioavailability and suppression of protective adaptive immune reactions [13, 14, 15 ]. Exuberant M2 TAM in breast cancers was strongly associated with poor prognosis, both in animal models and in patients [16-18].
A non-invasive diagnostic test, which can detect and quantify TAM non-invasively, could provide a novel prognostic assay for prediction of tumor progression and poor outcome in cancer patients and could be utilized to lead patients to individualized therapeutic options. Since immune-modulating cancer therapies have been translated to clinical practice, there is an immediate need for imaging tests that can non-invasively quantify macrophage responses in malignant tumors, both from the perspective of patient stratification and monitoring response to novel cancer immunotherapies [7]. Positron emission tomography (PET) and magnetic resonance imaging (MRI) are particularly suitable clinical imaging modalities for this purpose since PET radiotracers allow specific targeting with sensitive and quantitative detection of biomarkers, while MRI allows real-time assessment of modulating nanoparticles and imaging of endocytosis without additional radiation dose. We also consider published data on non-PET tracers since they could be adapted to PET by exchange of the isotope.
The purpose of our review article is to discuss the complementary role of TAM-directed radiotracers and iron oxide nanoparticles for monitoring cancer immunotherapies with PET and MRI technologies. In addition, we will outline how TAM-directed imaging and therapy is interdependent and can be interconnected towards improved clinical outcomes.
Macrophage targeting radiopharmaceuticals
Several radiotracers have been developed to target macrophages in vivo in the preclinical and clinical setting. In the past years, macrophage-targeted radiotracers have been mainly used to investigate inflammation-associated diseases including neuroinflammatory, rheumatoid and infectious diseases [19, 20]. More recently, a growing number of studies suggest the value of macrophage-specific radiotracers in the field of cancer diagnosis and therapy, which will be discussed in this section.
The high sensitivity of nuclear imaging, particularly PET, enables very low tracer doses, minimizing the effect on the biological system. However, radiopharmaceuticals can also affect macrophages, with the extent and outcome depending on the isotope used. Due to the plasticity of macrophage polarization markers, with overlapping and non-macrophage-restricted expression, it is challenging to develop specific molecular imaging tracers for specific TAM phenotypes. It is also important to recognize that the tracer material on its own can alter macrophage polarization. To evaluate tumor response to TAM modulating cancer treatments, it may be helpful to develop radiotracers that can distinguish between pro-tumorigenic and anti-tumorigenic macrophages.
The macrophage mannose receptor (MMR) CD206, the macrophage scavenger receptor CD163 and the folate receptor beta (FR-β) are the most distinctive surface markers for M2 differentiation ex vivo and therefore a primary subject of tracer development [21]. Targeting certain functional features such as phagocytic activity and antigen presentation is another focus and subject of endocytosis-associated and MHC class II (MHC-II) specific radiotracers. However, those functional markers may also be represented on other antigen-presenting cells and not specific for macrophages.
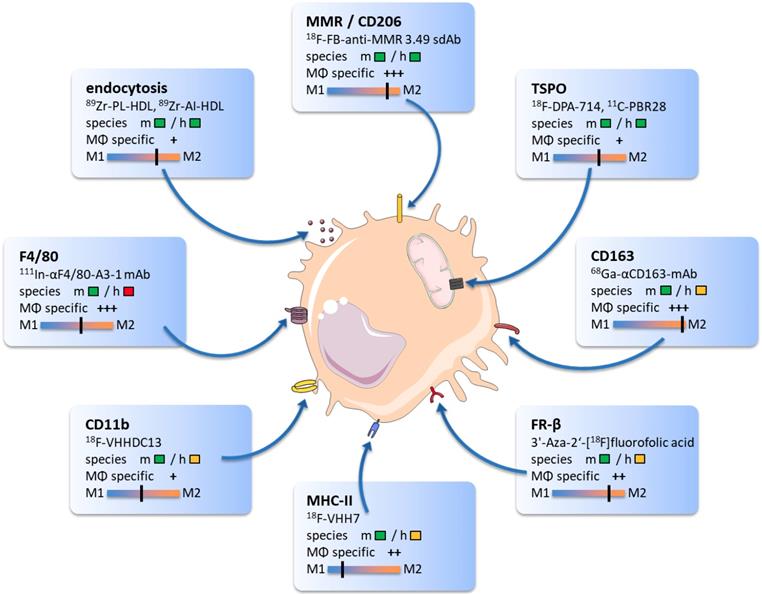
The following overview will highlight promising TAM biomarkers, their target specificity, and potential value for immunotherapy monitoring. We will provide examples of radiotracers that bind to particular proteins and discuss their ability to modify antitumoral therapies. The core findings of this section and related imaging examples are illustrated in Figure 1.
Translocator protein (TSPO)
The 18 kDa mitochondrial translocator protein (TSPO) has first been discovered in 1977 as an alternative binding site for diazepam in the kidneys [22]. TSPO is localized on the outer membrane of mitochondria and is involved in several critical cellular functions such as steroid synthesis, apoptosis and cell proliferation [23]. TSPO is primarily expressed in activated microglia, astrocytes, and infiltrating macrophages and is therefore a promising target for imaging inflammatory diseases [24].
There is evidence that TSPO is preferentially upregulated in M2 macrophages, but TSPO was also found on M0 and M1 macrophages, as well as other immune, stromal and endothelial cells [25]. Further, TSPO is upregulated in different cancer cells including brain, gastrointestinal, breast, and prostate tumors, potentially hampering its use for detection of TAM [26].
The use of TSPO-specific radiotracers has first been described in different neuroinflammatory diseases and brain injury [27]. While the first-generation TSPO radiotracer PK11195 suffers from a low signal-to-noise ratio due to its low brain permeability, nonspecific and plasma protein binding, second generation tracers are limited by overrepresentation of endothelial uptake in the blood-brain barrier and influence of the genetic background on the binding [28].18F-GE-180, a high-affinity third-generation radiofluorinated TSPO receptor ligand, demonstrated higher target-to-background ratios and allowed detection of gliomas in patients with a high tumor-to-background ratio [29]. However, it has not been elucidated to what extent the radiotracer signal in the tumor tissue was due to 18F-GE-180 uptake by glioma cells or TAM. More specific macrophage imaging has been shown in non-cancer mouse models of atherosclerosis by 18F-GE-180 [30], cardiac myocytes transplantation by 18F-DPA-714 [31], and tuberculosis using 125I-DPA-713 [32].
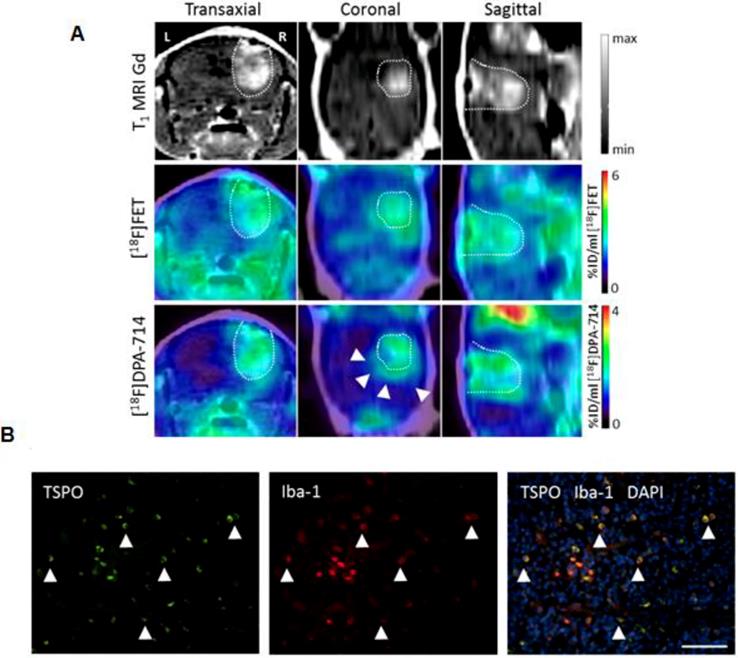
As one of the few TAM-focusing studies, Zinnhardt and colleagues identified specific compartments along mouse glioma margins with enhanced TSPO-specific 18F-DPA-714 uptake (Figure 2). Glioma-associated microglia/macrophages were identified as TSPO source [33]. In another preclinical study, Lanfranca et al. were able to track macrophage infiltration in a mouse model of pancreatic cancer with the PET tracer 11C-PBR28 [34]. Tracer uptake clearly correlated with histological macrophage tumor infiltration and specificity was proven in CD11b-deficient mice. However, the authors did not further analyze the subtypes of macrophages. TSPO is a sensitive marker for macrophages and TSPO tracers are clinically translatable [29], however they have not been shown yet to target M2 TAM specifically.
Macrophage mannose receptor (MMR, CD206)
The macrophage mannose receptor (CD206) is an endocytic carbohydrate-binding receptor expressed by selected populations of macrophages, dendritic cells and nonvascular endothelium. The roles attributed to this receptor include receptor-mediated endocytosis for MHC class II antigen presentation as well as modulation of cell activation and trafficking [35, 36]. MMR is preferentially expressed on M2 macrophages and has shown predictive value for cancer progression in different cancer types [37].
Mannose receptor targeting radiotracers were previously evaluated in mouse models of atherosclerosis [38, 39]. γ-Tilmanocept (Lymphoseek®), a 18 kDa mannose-decorated dextran labeled with 99mTc, was approved by the FDA for cancer sentinel lymph node detection [40, 41]. However, mannose and its analogues are not specific for CD206. Binding of other mannose receptors, such as CD209 expressed in the skin as well as intestinal and genital mucosa was reported [42, 43].
The graphic comprises promising targets for TAM imaging and related radiotracers. Target reactivity for mouse (m) and human (h) species is classified as fully developed tracer (  ), tracer development possible (
), tracer development possible (  ) and no species specific target expression (
) and no species specific target expression (  ). Tracer specificity for selective targeting of macrophages (MΦ) is listed for each target ranging from low (+) to high (+++) macrophage specific signal. Target specificity towards M1 or M2 phenotype was estimated based on existing literature and marked as black line.
). Tracer specificity for selective targeting of macrophages (MΦ) is listed for each target ranging from low (+) to high (+++) macrophage specific signal. Target specificity towards M1 or M2 phenotype was estimated based on existing literature and marked as black line.
Multi-tracer and multi-modality imaging for the characterization of the glioma immune microenvironment. (A) Gadolinium enhanced T1 weighted (T1 MRI Gd) of a human glioma model. PET-MR images for [18F]fluoroethyl tyrosine and the TSPO tracer [18F]DPA-714 [30] provide complementary information of heterogeneous glioma tissues (arrows). (B) Immunofluorescence analysis reveals tumor-associated macrophages/microglia (Iba-1, red) as important source of TSPO (green) in this model. Scale bar: 50 µm. Image courtesy of: B. Zinnhardt, C. Foray, C. Barca, O. Grauer, M. Schäfers and A. H. Jacobs, unpublished.
To enable more specific targeting, Zhang and colleagues used a radiolabeled anti-CD206 monoclonal antibody for non-invasive imaging of M2 macrophages, which served as an early biomarker for tumor relapse and lymph node metastasis in a murine breast cancer mouse model [44]. The groups of Devoogdt, Ginderachter and Caveliers generated single domain antibody fragments derived from camelids with high-affinity binding to CD206. These nanobodies with circulation and tissue penetration characteristics optimized for imaging were labeled with 99mTc and 18F. The researchers demonstrated that macrophages in the tumor stroma were specifically targeted by the CD206 nanobodies [45, 46]. In vivo PET/CT images of 18F are shown in Figure 3. Notably, the authors selected cross-species reactive nanobodies, which bind both to the mouse and human CD206 homologue with high affinity. This strategy allows direct translation from preclinical evaluation to first clinical studies using the same compound. Hence, CD206 targeting nanobodies are promising candidates for clinical imaging of M2-polarized macrophages.
Folate receptor β (FR-ß)
Folate receptors (FR) are glycosylphosphatidyl (GPI)-anchored plasma membrane proteins that bind folate and folic acids. Folate plays a complex role in the prevention and progression of cancer: reduced folate taken up by normal cells can prevent tumor development by supporting DNA repair of normal cells [47]. However, pre-neoplastic cells upregulate FR as their major and distinct route for endocytosis of non-reduced folate into the cell [47-50]. FR density on tumor cells increased as the cancer progressed and was associated with poor outcome in women with breast cancer [49-53].
There are several FR isoforms (α, β, and γ). The α isoform is over-expressed on the membrane surface of cancer cells and serves as a promising target for molecular therapies [54]. In contrast, the folate receptor beta (FR-β) is mainly restricted to myeloid cells. It is expressed by tumor-associated macrophages and is a marker for M-CSF induced M2 anti-inflammatory macrophages [55].
Transverse (upper row) and coronal (middle row) PET/CT images of wild-type (left) and MMR-deficient (right) 3LL-R tumor-bearing mice 3 h after injection of 18F-FB-anti-MMR3.49. PET signals are encoded in color scale, CT image in gray scale. Arrows point to tumor (T), kidney (K), and bladder (B). Autoradiography performed on slices from 3LL-R tumors grown in WT (left) vs. MMR-deficient (right) mice are shown in the bottom row. max = maximum; min = minimum. Reproduced after permission from [45]. Copyright © 2015 Society of Nuclear Medicine and Molecular Imaging.
FR-β as a macrophage-specific imaging target has been investigated preclinically in rheumatoid arthritis [56] and in clinical studies of patients suffering from atherosclerosis [57] or chronic obstructive pulmonary disease (NCT03494114). Moreover, we identified two single-photon emission computerized tomography (SPECT) studies using radiolabeled folate [58, 59] and one PET study with 3′-aza-2′-18F-fluorofolic acid in tumors [60]. However, the authors used FR-α expressing tumors, and folate analogues are not FR-ß selective. Thus, it could not be differentiated whether the tumor uptake of the tracers was derived specifically by the macrophages or by the tumor cells. It has further been shown that FR-ß is also expressed on certain human cancer cells, which might limit clinical applicability as a macrophage specific tracer [61].
Macrophage scavenger receptor CD163
The macrophage scavenger receptor CD163 is a high-affinity binder to the hemoglobin-haptoglobin complex and functions as a sensor for bacteria [62, 63]. In contrast to the aforementioned targets, CD163 is thought to be restricted to the monocytic-macrophage lineage, which makes it a very attractive imaging biomarker for these cells [64, 65]. CD163 is seen as one of the most reliable markers for M2-polarized macrophages and a proven predictive marker for tumorigenesis [21, 66].
PET imaging of a 68Ga labeled antibody for CD163 has been performed in rats with collagen-induced arthritis [67], but to our knowledge not yet in cancer models. Thus, CD163 is a very promising target for imaging of M2 macrophages but there is a lack of cancer specific evaluation and validation. Additionally, a soluble form of CD163 has been identified that is shed by the protease ADAM17 in humans but not in mice [68]. This has to be considered before its application towards clinical translation.
Active endocytosis
Macrophages are major phagocytic cells that engulf pathogens and present their peptide fragments to helper T cells. Apart from pathogens, nanoparticles and liposomes are preferentially phagocytosed by macrophages, monocytes, dendritic cells, and neutrophils [69].
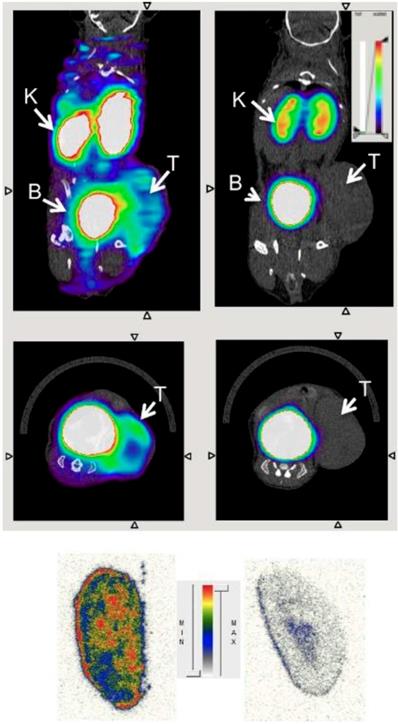
Macrophage-directed nanoparticles have been preferentially developed for MRI imaging. This is because macrophage phagocytosis takes several hours. To image tumor associated macrophages with MRI, we inject nanoparticles intravenously on day 1, wait for nanoparticle tumor perfusion, extravasation and phagocytosis and then image nanoparticles in TAM on day 2. Corresponding imaging techniques with PET or SPECT would require radiotracers with long half-lives such as 64Cu and 89Zr and hence, would be associated with high radiation exposure. To note, dextranated and DTPA-modified magneto-fluorescent 20-nm nanoparticle was labeled with the PET isotope 64Cu to allow preclinical PET/CT Imaging of macrophages in inflammatory atherosclerosis [70]. Pérez-Medina and colleagues further showed that 89Zr-labeled high-density lipoprotein nanoparticles composed of phospholipids and apolipoprotein A-I preferentially targeted TAM [71]. Respective PET/CT images are shown in Figure 4.
Other targets
EGF-like module-containing mucin-like hormone receptor-like 1 (EMR1), better known as F4/80, is a mouse-specific pan macrophage marker widely used as target in flow cytometry and fluorescence microscopy. Terry et al. developed an 111In-anti-F4/80 antibody to detect macrophages in spleen and tumors [72]. However, the characteristic F40/80 macrophage surface antigen is mouse specific. Its human homologue EMR1 is an eosinophil-specific marker and not suitable for macrophage imaging in the clinics [73].
Nanobodies specific for mouse class II MHC (MHC II) and CD11b were created and labeled with 18F and 64Cu by the group of Ploegh to detect myeloid cells in tumors and lymphoid organs. CD11b is a pan monocytic marker whereas MHC II is expressed on antigen presenting cells such as dendritic cells, B cells and M1 macrophages. The authors were able to visualize myeloid cell infiltration in a syngeneic and xenograft melanoma mouse model [74].
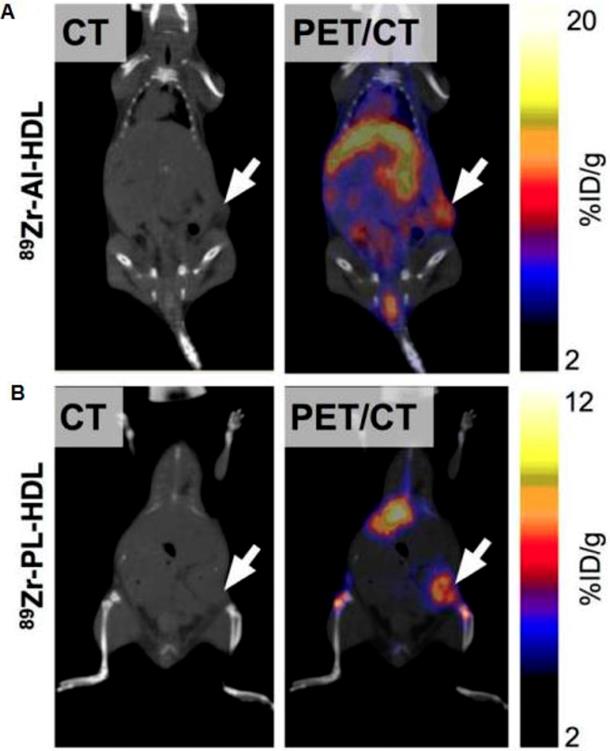
Visualisation of 89Zr-HDL nanotracer accumulation in tumor tissues by in vivo PET imaging. CT and PET/CT fusion sections of 89Zr-AI-HDL (A) and 89Zr-PL-HDL (B) obtained 24 h after injection in mice bearing orthotopic 4T1 tumors (indicated by arrows). Reproduced after permission from [71]. Copyright © 2015 Society of Nuclear Medicine and Molecular Imaging.
Notably, in contrast to all aforementioned tracers, the mouse MHC II radiotracer targets preferentially M1 macrophages. However, MHC II is expressed on a variety of different cell populations such as B cells and dendritic cells, which makes uptake values difficult to interpret. Furthermore, clinical translation is inherently challenging for antibody-based tracers since they often lack cross-reactivity between species and often need to be developed specifically for the species in question. In the case of antibodies for MHC the polygenic and polymorphic nature of the genetic locus further complicates development of a generally applicable probe.
The landscape of further potential biomarkers and tracers for macrophages goes far beyond the scope of this review. Substantial work on macrophage imaging has been done in the field of inflammation and atherosclerosis. Amongst others, SLC18B1 [75], iNOS [76], system xc- [77], somatostatin receptor [78], the chemokine receptor type (CXCR4) [79], and cysteine cathepsins [80, 81] have been successfully explored for macrophage imaging (including optical imaging) but either lack suitable PET tracers or await validation for cancer-associated macrophages. An extensive list of established radiotracers in the preclinical and clinical setting with focus on inflammatory diseases is available in the review of Jiemy and colleagues [19].
Image-guided macrophage-targeting therapies
Novel immunotherapies can either suppress tumor promoting M2 TAM or activate M1 TAM to attack and kill tumor cells. Tumor growth and metastasis formation can be decreased by TAM depletion, by inhibition of TAM recruitment and pro-tumoral function or by reprogramming TAMs into a pro-inflammatory M1 phenotype [82, 83]. TAM imaging can help to stratify patients with TAM-rich tumors to TAM-modulating therapies and monitor response to these therapies.
Macrophage targeting antibodies and other molecules are also used for targeted drug delivery. Examples are saporin toxin antibodies that bind to the anti-scavenger receptor A (CD204) [84] and FR-β binding immunotoxins conjugated to Pseudomonas exotoxin [85]. Especially the M2-specific targets such as CD206 and CD163 are promising candidates for specific modulation, inhibition or depletion of macrophages [86-88]. Receptor quantification and dose estimation to targeted drug and radionuclide delivery by PET using the respective radiotracers would enable treatment stratification and therapy response prediction [89]. This also accounts for PET imaging of the anti-phagocytic CD47 molecule expressed on cancer cells to estimate outcome of SIRPα/CD47-blocking antibody therapies [90, 91].
There is emerging evidence of radiation to have impact on antitumoral immune responses by release of tumor antigens, inflammatory signals and immune cell infiltration [92, 93]. Macrophage polarization can be influenced by external beam radiation. However, this effect seems to be dose-dependent and might also enhance invasive capability of tumor cells [94, 95].
Radiation dose of PET tracers are usually far below the therapeutic window, but therapeutic radionuclides such as 177Lu or 212Bis bound to target-specific pharmaceuticals can deliver relatively high doses to the target cells [96, 97]. First preclinical studies have shown synergistic effects of a vla-4-targeted radionuclide therapy and checkpoint blockade on immune cell infiltration and therapy response in a melanoma mouse model [98]. A clinical phase 1b study is underway to evaluate 177Lu-PSMA-617 and the immune checkpoint blocking antibody Pembrolizumab (NCT03805594).
In a theranostic setting, therapeutic radionuclides targeting CD206 or CD163 could reduce the number of pro-tumorigenic M2 TAMs, or potentially differentiate M2 TAMs into a M1 polarized phenotype. Although there is no data showing direct radiation effects on TAMs by radiopharmaceuticals, we believe this opens a promising new field in nuclear medicine.
Despite the ongoing developments in cancer treatment especially in the field of immune oncology, it is still evident that the majority of patients suffer from primary or acquired therapy resistance often mediated by immunosuppressive macrophages. The aforementioned therapy approaches emphasize the need of dedicated imaging techniques to detect and quantify different TAM populations with the goal to improve therapy outcome of cancer patients.
Monitoring cancer immunotherapy with iron oxide nanoparticles
Molecular imaging techniques for cancer imaging have largely focused on imaging cancer cells, cancer cell surface markers, tumor angiogenesis or the extracellular matrix [99-101]. However, the inflammatory component of the cancer microenvironment has not been a major target of imaging technologies for magnetic resonance imaging (MRI) thus far. Inflammatory macrophages have been imaged with nanoparticle-enhanced MRI in other inflammatory conditions, such as atherosclerosis and arthritis [102-105]. Inflammatory macrophages in malignant tumors have been targeted with preclinical imaging probes for combined fluorescence and MR imaging [106], 89Zr-labeled reconstituted high-density lipoprotein (rHDL) nanoparticles [71] and 64Cu-labeled mannosylated liposomes (MAN-LIPs) for PET imaging [107], a multimodality probe for fluorescence imaging, MRI and intravital microscopy [108], 99mTc-labeled anti-MMR (macrophage mannose receptor) nanobodies for single-photon emission computed tomography (SPECT)/micro-CT [46], Cy7-labeled deoxymannose for near-infrared fluorescence imaging [109] and bacterial magnetic nanoparticles for MRI detection [110]. While all of these approaches successfully detected TAM in cancers, they had the limitation that they were not clinically translatable due to in vivo toxicities or lack of biodegradation/elimination from the body [108, 111]. Several studies previously demonstrated that TAMs can be tracked with MRI contrast agents, such as manganese (Mn) chelates, iron oxide nanoparticles, and fluorine 19 (19F) incorporated perfluorocarbon compounds (PFCs). [112-114]. Small molecular gadolinium chelates, which are used for clinical MR imaging applications are not phagocytosed by TAM due to their small size. To achieve TAM targeting, Gd-chelates were conjugated to antibodies, peptides or other targeting moieties including anti-CEA F(ab')2, (MAB) RA96, RGDK and ZD2 [115-118]. However, these TAM-targeted Gd-chelates provided a low sensitivity for MRI detection and were not suitable for clinical translation [119]. In addition, it is not clear if Gd-nanoprobes in macrophages are metabolized and eliminated from the body. This is problematic for clinical translation as interstitial Gd-chelates can cause an irreversible soft tissue fibrosis and sclerosis [120, 121]. Thus far, few studies have focused on clinically translatable imaging technologies that enable the detection of TAM in patients.
Ferumoxytol nanoparticles are the only nanoprobes currently available for clinical imaging of TAM in patients. Other clinically translatable nanoparticle compounds in different stages of clinical development include ferumoxtran-10 (Sinerem) [121] and Molday Iron Oxide nanoparticles [122]. The FDA-approved iron supplement ferumoxytol (Feraheme™) is currently the only nanoparticle compound that is FDA approved and readily clinically available as an imaging agent via an “off label” use. Ferumoxytol is composed of iron oxide nanoparticles used for intravenous treatment of patients with iron deficiency [123]. However, ferumoxytol nanoparticles also provide measurable signal changes on MRI and can therefore be used as an MR contrast agent [124]. Intravenously injected ferumoxytol nanoparticles initially distribute in the blood pool due to their large size. Unlike larger nanoparticles (>50 nm), ferumoxytol nanoparticles transiently escape phagocytosis in liver, spleen and bone marrow, which leads to prolonged blood half-life and leaking across hyperpermeable tumor microvessels. The nanoparticles slowly accumulate in the interstitium of malignant tumors, where they are phagocytosed by TAM. This phagocytosis is a slow process, requiring delayed imaging for macrophage depiction at 24 hours after iron oxide injection [102-105]. At 24 hours postcontrast, experimental data revealed a specific cellular uptake and MR enhancement of ferumoxytol in TAM isolated from adenocarcinomas [125, 126]. No or minimal ferumoxytol uptake was noted in cancer cells [125]. The differential high ferumoxytol uptake by TAM and low or absent uptake by cancer cells is the basis for successful TAM imaging. Several studies in animal models and patients have shown that ferumoxytol nanoparticles are compartmentalized in TAMs at 24 hours post injection (p.i.). On these 24 hour delayed scans, the negative (dark) tumor enhancement on T2-weighted MR imaging studies correlated with TAM distribution on histopathology [125]. Cellular uptake of iron oxide nanoparticles led to a decreasing T1-signal effect, but persistent T2-signal effect on MR images [127]. This “decoupling” of T1- and T2-signal effects on MR images was indicative of intracellular compartmentalization. Recently, this concept has been translated to first-in-human clinical trials and showed that ferumoxytol-MRI can quantify TAM quantities in patients with glioblastoma [128], osteosarcoma and lymphoma [126]. Within each tumor group, T2* signal enhancement on MR images correlated significantly with the density of CD68+ and CD163+ TAM (P < 0.05) [126, 128].
M2 TAM in breast cancer directly correlated with tumor aggressiveness, and indirectly correlated with clinical outcome [7, 17, 129-131]. Preclinical and clinical data have shown that patients whose cancers are heavily infiltrated with TAMs benefit from combining chemotherapy with M2 TAM-antagonist therapeutics. TAM-selective imaging would facilitate identification of this patient population and enable monitoring patients during therapy. In mouse models of breast cancer, blocking TAM infiltration significantly enhanced efficacy of standard-of-care chemotherapy and extended overall survival [83, 132]. Daldrup-Link and team showed in preclinical models of breast cancer, that treatment with CSF1 (colony stimulating factor 1) monoclonal antibodies significantly reduced ferumoxytol tumor enhancement on delayed T2-weighted MR images and that this effect correlated with a significant decline in TAM quantities in the tumor tissue on histopathology, as determined by CD68 immuno stains and flow cytometry analyses [125]. These data indicated that tumor MR imaging with clinically applicable iron oxide nanoparticles enabled non-invasive quantification of TAM in neoplastic tissue where their presence serves as a novel biomarker for tumor therapy. Since clinical trials of new therapeutic drugs are expensive and take years to complete, the immediate value and impact of this new imaging approach could be immense.
Growth rate and MRI enhancement of human glioblastomas in representative NOD-SCID x RAG g/d dko mice. (A) Representative MR images with superimposed T2* relaxation time maps of anti-CD47 mAb treated tumor (upper row) and sham-treated control (lower row). (B) Corresponding tumor growth rates, calculated as % change in tumor size, and (C) T2* relaxation times of anti-CD47 mAb treated tumors (n=3) and sham-treated controls (n=5), displayed as means and standard deviations. Please note that ferumoxytol-induced T2*-signal enhancement is quantified by shortened T2* relaxation times (shorter T2* time = stronger contrast enhancement).
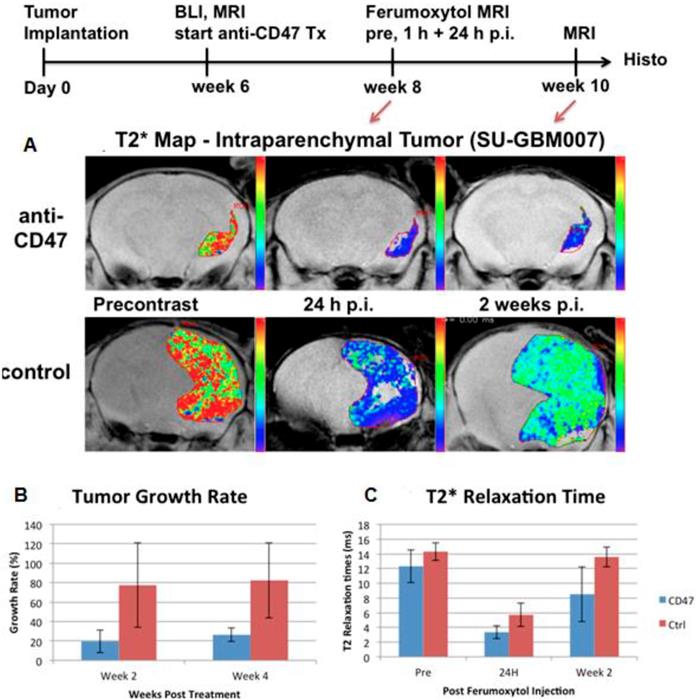
Of note, more than 90% of macrophages in malignant tumors represent M2 TAM. Therefore, for untreated tumors, there are limited applications of M1 TAM markers. However, cancer immunotherapy can either suppress M2 TAM or activate M1 TAM. A new TAM-directed immunotherapy approach is to reprogram tumor promoting M2 TAM phenotypes into tumor fighting M1 TAM phenotypes [95]. Work in the Weissman lab at Stanford has for the first time demonstrated that anti-cancer activity from the innate immune system can be activated via blockade of the immune-suppressive cell surface molecule CD47 expressed on tumor cells [133]. CD47 was expressed on the surface of all cancer cells analyzed, and CD47 blockade resulted in activation of anti-cancer activity from macrophages and eradication of tumors in mice [133]. This M1-TAM activation lead to increased tumor uptake of non-targeted nanoparticles and this effect could be imaged with MRI: Ferumoxytol-MRI was used to monitor response to anti-CD47 mAb therapy in mouse models of glioblastomas (Figure 5) and osteosarcomas [134], as noted by an increasingly negative (dark) nanoparticle enhancement of the tumor tissue compared to pre-treatment scans [134]. CD47 mAb cancer immunotherapies have been translated to the clinic and first-in-human Phase I/II clinical trials in patients are currently ongoing. A major side effect of anti-CD47 treatment is anemia. Ferumoxytol is a FDA-approved iron supplement for anemia treatment and might counteract this side effect. CD47 mAb-mediated macrophage polarization towards M1-TAM phenotypes would benefit from specific imaging biomarkers for M1-TAM. However, to our knowledge there are no clinically translatable M1-specific TAM markers for MR imaging to date.
It is important to note that standard chemotherapies and immune modulating therapies do not represent a binary concept. Many standard chemotherapeutic agents have intrinsic immune modulating properties, which are important to understand and consider with planned new combination therapies. For example, Doxorubicin is established for the treatment of osteosarcomas and acts on a common mechanistic pathway with CD47 mAb by inducing immunogenic cell death [135]. Doxorubicin induces the expression of calreticulin on the cell surface of sarcoma cells that binds to low-density lipoprotein receptor-related protein 1 (LRP1) and functions as a pro-phagocytic “eat me” signal for TAM [136, 137]. We have recently shown that this effect can be visualized with ferumoxytol-MRI. Osteosarcomas in mouse models demonstrated significantly stronger ferumoxytol enhancement and significantly increased TAM quantities after CD47 mAb plus doxorubicin combination therapy compared to CD47 mAb (P = 0.02) and doxorubicin monotherapy (P = 0.001) [186]. Since cytotoxic drugs may be either immunostimulatory or immunosuppressive [138], ferumoxytol-MRI might be useful as a new tool to find synergistic drug combinations and recognize antagonistic combinations. While other iron oxide nanoparticles [139, 140] and other metal-doped nanoparticles [141] have been used to label macrophages, they are not clinically translatable and therefore, of uncertain clinical impact.
Limitations of ferumoxytol-enhanced MRI for TAM detection
Ferumoxytol doses up to 400 mg Fe/kg were non-lethal in rodents [142] and ferumoxytol nanoparticles were generally well tolerated by most patients. However, rare anaphylactic reactions have been described in adult patients [123, 143, 144]. Likewise, our initial experience with ferumoxytol administrations did not reveal any side effects. However, due to the risk of rare, but potentially severe allergic or pseudo-allergic reactions, it is important to screen patients for any history of allergies and use, proper, slow iron administration techniques to avoid iron-induced hypotensive reactions.
Makela et al tracked TAMs labeled with iron oxide nanoparticles and perfluorocarbon (PFC) agents with MRI in 4T1 breast tumors [145]. A signal loss of the entire tumor was observed after 4 days of iron oxide nanoparticle treatment and a more pronounced signal loss of the tumor periphery was noted at 3 weeks, indicating higher accumulation of TAMs in the tumor periphery at 3 weeks. However, after PFC administration, similar spatial fluorine-19 (19F) signal was noted in the tumor center and periphery, indicating the presence of similar TAM quantities in different tumor areas. This study suggested that 19F-based TAM tracking methods provide different information compared to the iron-based TAM imaging technology. In another report by Leftin et al, the authors reported that the depiction of TAMs in breast cancer models can be significantly enhanced by focusing on spatial distributions of iron deposits instead of ROI averages [146]. Previous studies showed that both M1- and M2-TAMs take up ferumoxytol. Thus, we are not able to discriminate these sub-populations with ferumoxytol. Nanoparticles that are directed to specific surface markers, such as mannose for the M2 subtypes, could provide more specific targeting in the future.
Recently, proliferating macrophages have been identified as an abnormal TAM subpopulation associated with high-grade cancers and increased risk of recurrence [131]. We would expect these metabolically active, proliferating TAMs to show marked nanoparticle uptake, while non-activated monocytes may show lesser or no ferumoxytol uptake. This theory will be evaluated by co-localization analysis of anti-dextran and CD68 stains, augmented with other cell-type specific immunostains [131].
Modulating effects of Iron Oxide Nanoparticles
Intrinsic effects of iron oxide nanoparticles on tumor associated macrophages
Iron oxide nanoparticles have gained interest for cancer imaging due to their relatively easy synthesis, small size, high surface to volume ratio, easy functionalization, and multifunctional theranostics capabilities [147-152]. The intrinsic effects of magnetic iron oxide nanoparticles on macrophages can occur in the following ways: a) NPs stimulate M1 polarization [153]. b) NPs induce ferroptosis [154].
Iron oxide nanoparticles are presently used as iron replacement therapies clinically [155]. Iron exposure can regulate iron transport-related proteins that are associated with macrophage polarization states [156]. Recent studies by Daldrup-Link and co-workers showed that ferumoxytol nanoparticles could suppress tumor growth by inducing M1 macrophage polarization in early mammary cancers, and lung cancer metastases in liver and lungs. [157]. Tumor cells co-injected with ferumoxytol exhibited a markedly delayed growth rate as compared to tumor cells injected without ferumoxytol [157]. Flow cytometry and histopathology showed that the observed tumor growth inhibition was accompanied by increased presence of pro-inflammatory M1 macrophages in the tumor tissues (Figure 6) [157]. Tumor sections obtained at day 7 after their implantation into experimental mice showed increased presence of CD80+ cells within ferumoxytol-co-implanted tumors compared to controls, apparently representing increased quantities of pro-inflammatory M1 macrophages. Previous in vitro studies showed that superparamagnetic iron oxides induce a phenotypic shift in M2 macrophages towards a high CD86+, TNFα positive M1 macrophage subtype [158]. In the presence of iron oxide nanoparticles, M1-TAM polarization can induce a Fenton reaction: Activated M1-TAM produce hydrogen peroxide, which reacts with ferrous iron to produce hydroxyl radicals that destroy organic material [159]. Cancer cells exposed to hydrogen peroxide and hydroxyl radicals produce oxidized lipids, proteins, and damaged DNA, which can induce cell death. Dying cancer cells produce high levels of reactive oxygen species (ROS), which are released into the extracellular area when the cellular membrane is degraded during cell death. Extracellular H2O2 serves as chemo-attractant to monocytes and drives monocyte-to-M1 macrophage polarization [160]. This continued M1-polarization can create an autocrine feedback loop that maintains the production of TNFα and nitric oxide and thereby, causes continued cancer cell death and tumor growth inhibition. These observations were confirmed in a second animal model of glioblastoma multiforme, where the intrinsic immune-modulatory effect was most effective in early stage tumors, similar to other approaches of cancer immunotherapy [161, 162]. Similarly, Zhao et al. recently reported that ferumoxytol nanoparticles induced pro-inflammatory macrophage polarization in aggressive melanoma cancers. Toll-like receptor 3 (TLR3) activation increased the anti-tumor response of immune cells by manipulating cytotoxic T lymphocytes (CTL) and anti-tumor natural killer (NK) cells through the maturation of dendritic cells (DCs) [163, 164]. Ferumoxytol combined with a TLR3 agonist, poly (I:C) (PIC), demonstrated synergistic inhibition of tumor growth by shifting macrophages to a tumoricidal phenotype [164]. This was also correlated with upregulation of TNF-α and iNOS, with an enhanced NO secretion. In a combination therapy of ferumoxytol with the TLR3 agonist PIC showed superior melanoma regression and anti-metastatic efficacy that is associated within filtration of pro-inflammatory macrophage response and upregulation of pro-inflammatory genes in vivo (Figure 7) [164]. These findings suggest that ferumoxytol nanoparticles hold great potential to macrophage-modulating cancer immunotherapy by inducing the tumor-suppressive macrophage polarization within the tumor microenvironment.
Ferroptosis is a newly illustrated programmed cell death mechanism that takes place via an iron and lipid peroxidation dependent process and is stimulated by glutathione diminution [165, 166]. Even though, iron is a promoter of cancer cell proliferation, it plays a crucial role for producing reactive oxygen species (ROS) and lipid peroxidation via the Fenton reaction. Iron metabolism consisting of iron uptake, reflux and storage can also induce ferroptosis, a distinct, iron-dependent type of programmed cell death characterized by the accumulation of lipid peroxides. Some studies suggest that iron-based NPs can induce ferroptosis of cancer cells [154]. Iron oxide nanoparticles which can be directed by magnetic fields might be particularly useful for ferroptosis-based cancer therapy. Zhou et al. showed an iron oxide nanoparticle based delivery of linoleic acid hydroperoxide (LAHP) polymers to transport Fe2+, generating ROS and 1O2 that has led to programmed cancer cell death or ferroptosis.[167]. Zhang et al. reported amorphous iron (Fe0) nanoparticles (AFeNPs) for ferroptosis-based cancer therapy in a breast cancer model. Mechanistic studies revealed that ionization of the AFeNPs facilitates ferrous ion release in the tumor, which led to H2O2 and hydroxyl radical generation (•OH or •OOH) [168]. Huan et al. demonstrated the assessment of zero-valent iron-based (ZVI NPs) nanotherapeutics for induced cancer cell death or ferroptosis and resensitization strategy based on ferroptosis inducers with minimal side effects to healthy non malignant cells [169].
Apart from ferumoxytol (Feraheme™), various other iron oxide-based nanoparticles including ferucarbotran and ferumoxides have been extensively used as clinically approved MRI contrast agents [170, 171]. Treating M2-polarized macrophages with ferucabotran in vitro caused an increase in the expression of CD86, ferritin, cathepsin L and M1-like polarization [158]. Costa da Silva et al. monitored the accumulation of iron in lung tissues from lung cancer patients [172]. Hemolysis-induced iron accumulation was inversely related with CD68 expression on TAM. Iron-containing macrophages demonstrated decreased expression of CD206 (with decrease in tumor size) and increased expression of CD86 and iNOS. Moreover, these macrophages showed an increase in IL-10 and IL-6 secretion indicating an M1-polarizing shift. These results support the functions of iron and hemolytic RBC for the repolarization of TAM to employ an anti-tumor effector function using an adjuvant therapeutic strategy to endorse an anti-cancer immune response.
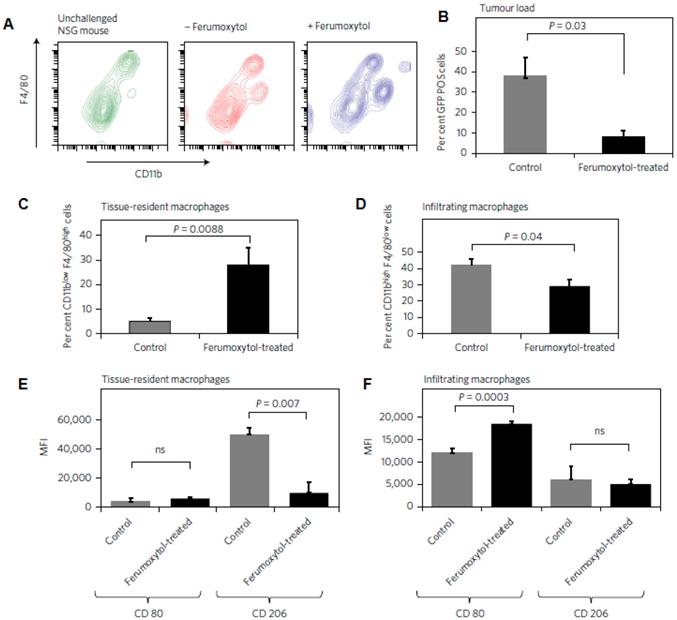
Ferumoxytol alters macrophage polarization in hepatic metastasis in vivo. a, Livers of the same mice described in Fig. 6 were further analysed with FACS for infiltrating leukocyte populations. b, Accordingly, the relative number of GFP+ cells within the liver gate (%) was significantly reduced in ferumoxytol-treated livers compared with untreated controls. c, CD11blowF4/80high tissue-resident macrophages were increased in ferumoxytol-treated livers compared to controls and d, CD11bhighF4/80low peripheral-derived macrophages were increased in ferumoxytol-treated livers compared with controls. e,f, The polarization of both tissue-resident and infiltrating macrophages shifted towards the M1 phenotype as measured by CD80 and CD206 markers: median fluorescent intensity ratios (MFI) of M1/M2 associated markers (CD80/CD206) in CD11blowF4/80high tissue-resident macrophages (e) and infiltrating F4/80low CD11bhigh liver macrophages (f) isolated from ferumoxytol-treated livers and untreated controls. All quantitative data are displayed as the mean of seven livers per group ± standard deviation. *P < 0.05, indicates a statistically significant difference (Student's t-test) from untreated controls. Reproduced after permission from [157]. Copyright © 2016 NPG.
In a recent published article, Chen et al. showed for the first time the use of iron oxide embedded mesoporous organosilica nanocomposite (IO-LPMONs), for the activation of cytotoxic T cells and polarization of macrophages in tumor immunotherapy [152]. These nanoparticles efficiently delivered OVA to DCs, activated DCs, which in turn activated both CD4+ and CD8+ effector antigen specific T cells and ultimately lead to strong anti-tumor effects. Additionally, the IO-LPMONs acted as an immune modulator for the polarization of TAMs from an immunosuppressive M2 to a tumor killing M1 phenotype, inducing efficient tumor apoptosis. This combination of macrophage polarization and T cell activation strategy induced potent anti-tumor effects in vivo in EG7-OVA tumor bearing C57BL6 mice. Kodali et al. compared the effects of superparamagnetic iron oxide nanoparticles (SPIONs) and silica nanoparticles (SiNPs) on lung macrophages by measuring differences in gene expressions [173]. SPION treatment altered a total of 1029 genes, while SiNPs altered the expression of 67 genes. SPION treatments increased TNF-α secretion and reduced IL-10 secretion from macrophages compared to SiNPs treatments. This showed the stronger M1-polarization effects of SPIONs compared to SiNPs.
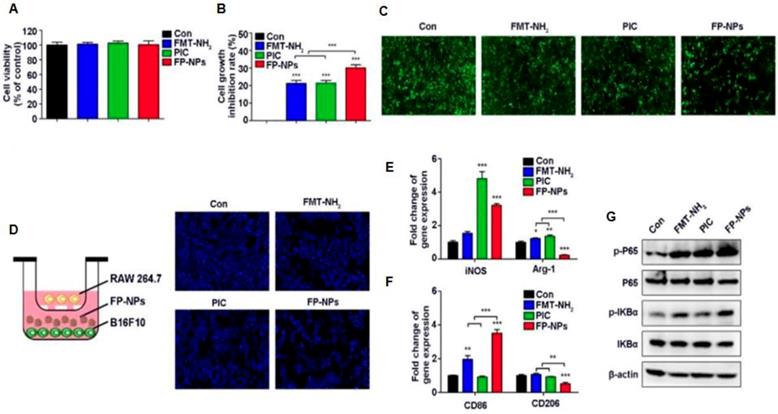
Effect of FP-NPs on tumor cell proliferation and macrophage polarization. (A) B16F10 cells were incubated with FMT-NH2, PIC, or FP-NPs for 48 h and the cell viability was analyzed by CCK-8 assay. (B) B16F10 cells pre-labeled with CFSE co-cultured with RAW 264.7 at a ratio of 2:1 were incubated with FMT-NH2, PIC, or FP-NPs for 48 h and cell proliferation was then analyzed by FCM. (C) GFP-B16F10 cells co-cultured with RAW 264.7 at a ratio of 2:1 were incubated with FMT-NH2, PIC, or FP-NPs for 48 h and the fluorescence intensity of GFP was captured. (D) Left: Schematic diagram of the co-culture. Right: B16F10 cells co-cultured with RAW 264.7 at a ratio of 2:1 were incubated with FMT-NH2, PIC, or FP-NPs in a dual-chamber Transwell system for 48 h and the tumor cells in the lower chamber were stained with DAPI and analyzed by fluorescence microscopy. (E, F) RAW 264.7 were incubated with FMT-NH2, PIC, or FP-NPs for 12 h and the expression of macrophage M1 (iNOS, CD86) and M2 (Arg-1, CD206) related genes was analyzed by qRT-PCR. (G) RAW 264.7 cells were incubated with FMT-NH2, PIC, or FP-NPs for 15 min and the expression of target proteins was analyzed by WB. All representative data are from three independent experiments. Error bars, SD. *P < 0.05, **P < 0.01, ***P < 0.001. FCM: flow cytometry; FMT: ferumoxytol; FP-NPs: FMT-NH2-poly I:C; iNOS: inducible nitric oxide synthase; PIC: poly I:C, polyinosinic-polycytidylic acid. WB: western blotting. Reproduced after permission from [164]. Copyright © 2018 Ivyspring International Publisher Pty Ltd.
SPIONs can also play a role in the generation of reactive oxygen species (ROS) which can induce pro-inflammatory cytokines and interleukins in the tumor microenvironment. Rojas et al. synthesized aminopropyl silane-, di-mercaptosuccinic acid- and amino dextran-coated SPIONs for the generation of ROS in TAM [174]. Mulens-Arias et al. reported that the treatment of macrophages by polyethyleneimine (PEI)-coated SPIONs amplified the expressions of ferritin, CD8 and CD86 along with the secretion of IL-12 and IL-10 from macrophages [175].
Alternating magnetic field (AMF) therapy has shown great promise for controlled drug release from iron oxide nanoparticles and for induction of tumor hyperthermia [176]. However, the effects of AMF on the macrophage polarization were not well studied. Kang et al. studied the effects of AMF therapy using the treatment of RGD peptide-amino-silica-coated SPIONs on macrophage polarization [177]. Following nanoparticle treatment, a low frequency AMF was applied on the mice that demonstrated increased arginase-1 and decreased iNOS in mice macrophages, supporting for M2-like polarization state. Further, high frequency AMF exposure exhibited an M1-like polarization state. Toraya-Brown et al. has demonstrated the application of magnetic hyperthermia by combining iron oxide nanoparticles and AMF [178]. The tumor hyperthermia of melanoma tumors activated the dendritic cells (DCs) and CD8+ T cells that promoted a strong resistance against reoccurrence of the melanoma cancer or metastasis. These recent studies thus support that the AMF therapy shows promise as a regulator for macrophage polarization and inducing anti-tumor immune responses using SPIONs therapy.
Engineering iron oxide nanoparticles to enhance immune-modulating effects on the cancer microenvironment
Engineering iron oxide nanoparticles to augment or supplement cancer immunotherapies has become an emerging area of research. This section will provide examples of this approach.
Tumor accumulation of T cells for immunotherapy can be enhanced by magnetic navigation of nanoparticle targeted T cells to tumors [179]. Mühlberger et al. demonstrated the magnetic navigation of T-cells, which were loaded with SPIONs and immune modulatory drugs [180]. They synthesized lauric acid (LA) and albumin coated SPIONs, incubated them with mouse cytotoxic T lymphocytes and attracted tumor accumulation of SPION loaded T cells with an external magnetic field. Perica et al. conjugated Major Histocompatibility Complex-Peptide and co-stimulatory anti-CD28 to paramagnetic iron-dextran coated SPIONs. The resulting artificial antigen-presenting cells (aAPCs) were able to bind to T cell receptors, capturing T-cells in a magnetic column and activating them [181]. This resulted in 1000-fold expansion of tumor-specific T cells in one week [181]. Zhang et al coated magnetic nanoclusters with azide-engineered leucocyte membranes and T-cell stimuli. The resultant multifunctional aAPCs stimulated CD8+ T cells and enabled in vivo tracking of intravenously injected T cells to tumors in mouse models with MRI [182]. Nanoscale aAPCs induced regression of tumor growth in an in vivo lymphoma model without observable toxicity.
SPIONs have been also used to generate nanovaccines, which train and stimulate the immune system to recognize and combat cancer cells. Cho et al. used multifunctional iron oxide-zinc oxide core-shell nanoparticles to deliver carcinoembryonic antigen into DCs, which induced an immune response and reduced tumor growth and improved mice survival [183]. The DCs were transfected ex vivo by the nanoparticle-antigen complex, injected into tumor-bearing mice and caused significant tumor antigen T cell response. The nanovaccine could be tracked in vivo with MRI. In another recent study, Zhao et al. used SPIONs to deliver Ovalbumin EndoFit (OVA) vaccine [184]. In comparison of OVA and SPIONs alone, the OVA-conjugated SPIONs demonstrated significantly increased immune responses and inhibition of tumor growth. The OVA formulated SPIONs showed the activation of immune cells and cytokine production, inducing significant cellular and humoral immune responses.
Shevtsov et al. demonstrated enhanced immunostimulatory activity of SPIONs coated with Hsp70, a heat shock protein. The authors showed the delivery of immunogenic peptides from tumors lysates to DCs by Hsp70-SPIONs that helped to stimulate tumor-specific T cell response and reduce tumor growth by enabling antigen trafficking to APCs [185].
Conclusion and future perspective
In summary, TAM play a fundamental dualistic role in carcinogenesis, tumor growth and metastasis. Immediately clinically available imaging techniques for in vivo detection of TAM in patients include ferumoxytol-enhanced MRI and PET imaging with translocator protein (TSPO)-targeted radiotracers. Future opportunities include the development of TAM biomarkers that are designed to more specifically target pro- and anti-tumoral macrophage phenotypes in the tumor microenvironment. Examples include Mannose-, CD206 and CD-163-targeted radiochemicals for specific imaging of M2-TAM phenotypes.
Furthermore, with the predominant existence of M2-polarized macrophages within the tumor environment, research attempts have almost exclusively focused on the development of M2-directed imaging agents. The emerging interest in macrophage modulating drug development implies the need of M1-targeting imaging techniques as well, for which only very few have been described so far [74].
While significant advances are being made in the development of TAM-specific imaging biomarkers for MRI and PET, future studies can leverage their complementary strengths. On the one hand, the simultaneous application of two complementary imaging agents allows increasing the accuracy and specificity of macrophage targeting. On the other hand, the combination of a macrophage imaging agent (e.g. ferumoxytol-MRI) with further immune cell targeting agents such as the novel T cell specific immunotracers will extend the ability to display complex immune responses by in vivo imaging.
Challenges will include the preservation of the high sensitivity of current standard imaging tests for tumor detection while adding specificity. For example, combined tumor detection and TAM quantification could be achieved by combining clinical standard FDG-PET with ferumoxytol MRI or by combining whole body diffusion weighted MRI with TAM-specific PET imaging. More detailed diagnoses of immunotherapy induced changes in M1/M2 TAM compositions of the tumor microenvironment could be achieved by combining ferumoxytol MRI for imaging the total TAM population with radiotracers for imaging M2-TAM. Similarly, the TAM-directed diagnostic probes could be loaded with therapeutic drugs to generate theranostic (combined diagnostic and therapeutic) probes that will enable image-guided, personalized therapies.
Abbreviations
AMF: alternating magnetic field; CT: computed tomography; EMR1: EGF-like module-containing mucin-like hormone receptor-like 1; FR: folate receptor; IL: interleukin; IONP: iron oxide nanoparticles; IO-LPMON: iron oxide-embedded large-pore mesoporous organosilica nanospheres; M1: macrophages with an inflammatory, antitumoral phenotype; M2: macrophages with an anti-inflammatory, protumoral phenotype; mAb: monoclonal antibody; MHC II: major histocompatibility complex class II; MMR: macrophage mannose receptor; MΦ: macrophage; MRI: magnetic resonance imaging; NP: nanoparticle; PET: positron emission tomography; sdAb: single domain antibody; SiNP: silica nanoparticle; SPECT: single photon emission computed tomography; SPION: superparamagnetic iron oxide nanoparticle; TAM: tumor associated macrophages; TNF-α: tumor necrosis factor alpha; TSPO: mitochondrial translocator protein.
Acknowledgements
This manuscript was in part supported by a grant from the National Institute of Child Health and Human Development (NICHD), grant number R01HD081123, to HDL. Illustrations of cellular components (modified), therapeutics and imaging modalities in the graphical abstract and figure 1 are derived from Servier Medical Art (https://smart.servier.com), licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0). Dr. Sudip Mukherjee acknowledges Dr. Omid Veiseh for providing postdoctoral mentorship and support.
Competing Interests
The authors have declared that no competing interest exists.
References
1. DeNardo DG, Coussens LM. Inflammation and breast cancer - Balancing immune response: crosstalk between adaptive and innate immune cells during breast cancer progression. Breast Cancer Res. 2007;9:212
2. Gonzalez H, Hagerling C, Werb Z. Roles of the immune system in cancer: from tumor initiation to metastatic progression. Genes Dev. 2018;32:1267-84
3. van Dalen FJ, van Stevendaal M, Fennemann FL, Verdoes M, Ilina O. Molecular Repolarisation of Tumour-Associated Macrophages. Molecules. 2018;24:9
4. Siveen KS, Kuttan G. Role of macrophages in tumour progression. Immunol Lett. 2009;123:97-102
5. Solinas G, Germano G, Mantovani A, Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol. 2009;86:1065-73
6. Poh AR, Ernst M. Targeting Macrophages in Cancer: From Bench to Bedside. Front Oncol. 2018;8:49
7. Mukhtar RA, Nseyo O, Campbell MJ, Esserman LJ. Tumor-associated macrophages in breast cancer as potential biomarkers for new treatments and diagnostics. Expert Rev Mol Diagn. 2011;11:91-100
8. Bjerkvig R, Tysnes BB, Aboody KS, Najbauer J, Terzis AJ. Opinion: the origin of the cancer stem cell: current controversies and new insights. Nat Rev Cancer. 2005;5:899-904
9. Yang L, Zhang Y. Tumor-associated macrophages: from basic research to clinical application. J Hematol Oncol. 2017;10:58
10. Lin Y, Xu J, Lan H. Tumor-associated macrophages in tumor metastasis: biological roles and clinical therapeutic applications. J Hematol Oncol. 2019;12:76
11. Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7:211-7
12. Salmaninejad A, Valilou SF, Soltani A, Ahmadi S, Abarghan YJ, Rosengren RJ. et al. Tumor-associated macrophages: role in cancer development and therapeutic implications. Cell Oncol (Dordr). 2019 doi: 10.1007/s13402-019-00453-z
13. Kumar V, Gabrilovich DI. Hypoxia-inducible factors in regulation of immune responses in tumour microenvironment. Immunology. 2014;143:512-9
14. Lin EY, Li JF, Gnatovskiy L, Deng Y, Zhu L, Grzesik DA. et al. Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res. 2006;66:11238-46
15. Crowther M, Brown NJ, Bishop ET, Lewis CE. Microenvironmental influence on macrophage regulation of angiogenesis in wounds and malignant tumors. J Leukoc Biol. 2001;70:478-90
16. Sousa S, Brion R, Lintunen M, Kronqvist P, Sandholm J, Monkkonen J. et al. Human breast cancer cells educate macrophages toward the M2 activation status. Breast Cancer Res. 2015;17:101
17. Leek RD, Lewis CE, Whitehouse R, Greenall M, Clarke J, Harris AL. Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Res. 1996;56:4625-9
18. Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71-8
19. Jiemy WF, Heeringa P, Kamps J, van der Laken CJ, Slart R, Brouwer E. Positron emission tomography (PET) and single photon emission computed tomography (SPECT) imaging of macrophages in large vessel vasculitis: Current status and future prospects. Autoimmun Rev. 2018;17:715-26
20. Foss CA, Sanchez-Bautista J, Jain SK. Imaging Macrophage-associated Inflammation. Semin Nucl Med. 2018;48:242-5
21. Vogel DY, Glim JE, Stavenuiter AW, Breur M, Heijnen P, Amor S. et al. Human macrophage polarization in vitro: maturation and activation methods compared. Immunobiology. 2014;219:695-703
22. Braestrup C, Squires RF. Specific benzodiazepine receptors in rat brain characterized by high-affinity (3H)diazepam binding. Proc Natl Acad Sci USA. 1977;74:3805-9
23. Denora N, Natile G. An Updated View of Translocator Protein (TSPO). Int J Mol Sci. 2017;18:2640
24. Papadopoulos V, Baraldi M, Guilarte TR, Knudsen TB, Lacapere JJ, Lindemann P. et al. Translocator protein (18kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol Sci. 2006;27:402-9
25. Narayan N, Owen DR, Mandhair H, Smyth E, Carlucci F, Saleem A. et al. Translocator Protein as an Imaging Marker of Macrophage and Stromal Activation in Rheumatoid Arthritis Pannus. J Nucl Med. 2018;59:1125-32
26. Batarseh A, Papadopoulos V. Regulation of translocator protein 18 kDa (TSPO) expression in health and disease states. Mol Cell Endocrinol. 2010;327:1-12
27. Guilarte TR. TSPO in diverse CNS pathologies and psychiatric disease: A critical review and a way forward. Pharmacol Ther. 2019;194:44-58
28. Vivash L, O'Brien TJ. Imaging Microglial Activation with TSPO PET: Lighting Up Neurologic Diseases? J Nucl Med. 2016;57:165-8
29. Albert NL, Unterrainer M, Fleischmann DF, Lindner S, Vettermann F, Brunegraf A. et al. TSPO PET for glioma imaging using the novel ligand F-18-GE-180: first results in patients with glioblastoma. Eur J Nucl Med Mol Imaging. 2017;44:2230-8
30. Hellberg S, Liljenback H, Eskola O, Morisson-Iveson V, Morrison M, Trigg W. et al. Positron Emission Tomography Imaging of Macrophages in Atherosclerosis with (18)F-GE-180, a Radiotracer for Translocator Protein (TSPO). Contrast Media Mol Imaging. 2018;2018:9186902
31. Kashiyama N, Miyagawa S, Fukushima S, Kawamura T, Kawamura A, Yoshida S. et al. Development of PET Imaging to Visualize Activated Macrophages Accumulated in the Transplanted iPSc-Derived Cardiac Myocytes of Allogeneic Origin for Detecting the Immune Rejection of Allogeneic Cell Transplants in Mice. PLoS One. 2016;11:e0165748
32. Foss CA, Harper JS, Wang H, Pomper MG, Jain SK. Noninvasive molecular imaging of tuberculosis-associated inflammation with radioiodinated DPA-713. J Infect Dis. 2013;208:2067-74
33. Zinnhardt B, Pigeon H, Theze B, Viel T, Wachsmuth L, Fricke IB. et al. Combined PET Imaging of the Inflammatory Tumor Microenvironment Identifies Margins of Unique Radiotracer Uptake. Cancer Res. 2017;77:1831-41
34. Lanfranca MP, Lazarus J, Shao X, Nathan H, Di Magliano MP, Zou W. et al. Tracking Macrophage Infiltration in a Mouse Model of Pancreatic Cancer with the Positron Emission Tomography Tracer [11C]PBR28. J Surg Res. 2018;232:570-7
35. Martinez-Pomares L. The mannose receptor. J Leukoc Biol. 2012;92:1177-86
36. Gazi U, Martinez-Pomares L. Influence of the mannose receptor in host immune responses. Immunobiology. 2009;214:554-61
37. Liu DR, Guan QL, Gao MT, Jiang L, Kang HX. Mannose receptor as a potential biomarker for gastric cancer: a pilot study. Int J Biol Markers. 2017;32:E278-E83
38. Tahara N, Mukherjee J, de Haas HJ, Petrov AD, Tawakol A, Haider N. et al. 2-deoxy-2-[18F]fluoro-D-mannose positron emission tomography imaging in atherosclerosis. Nat Med. 2014;20:215-9
39. Varasteh Z, Hyafil F, Anizan N, Diallo D, Aid-Launais R, Mohanta S. et al. Targeting mannose receptor expression on macrophages in atherosclerotic plaques of apolipoprotein E-knockout mice using (111)In-tilmanocept. EJNMMI Res. 2017;7:40
40. Azad AK, Rajaram MV, Metz WL, Cope FO, Blue MS, Vera DR. et al. gamma-Tilmanocept, a New Radiopharmaceutical Tracer for Cancer Sentinel Lymph Nodes, Binds to the Mannose Receptor (CD206). J Immunol. 2015;195:2019-29
41. Marcinow AM, Hall N, Byrum E, Teknos TN, Old MO, Agrawal A. Use of a Novel Receptor-Targeted (CD206) Radiotracer, Tc-99m-Tilmanocept, and SPECT/CT for Sentinel Lymph Node Detection in Oral Cavity Squamous Cell Carcinoma Initial Institutional Report in an Ongoing Phase 3 Study. JAMA Otolaryngol Head Neck Surg. 2013;139:895-902
42. Geijtenbeek TB, Torensma R, van Vliet SJ, van Duijnhoven GC, Adema GJ, van Kooyk Y. et al. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell. 2000;100:575-85
43. Jameson B, Baribaud F, Pohlmann S, Ghavimi D, Mortari F, Doms RW. et al. Expression of DC-SIGN by dendritic cells of intestinal and genital mucosae in humans and rhesus macaques. J Virol. 2002;76:1866-75
44. Zhang CR, Yu XH, Gao LQ, Zhao Y, Lai JH, Lu DH. et al. Noninvasive Imaging of CD206-Positive M2 Macrophages as an Early Biomarker for Post-Chemotherapy Tumor Relapse and Lymph Node Metastasis. Theranostics. 2017;7:4276-88
45. Blykers A, Schoonooghe S, Xavier C, D'Hoe K, Laoui D, D'Huyvetter M. et al. PET Imaging of Macrophage Mannose Receptor-Expressing Macrophages in Tumor Stroma Using 18F-Radiolabeled Camelid Single-Domain Antibody Fragments. J Nucl Med. 2015;56:1265-71
46. Movahedi K, Schoonooghe S, Laoui D, Houbracken I, Waelput W, Breckpot K. et al. Nanobody-based targeting of the macrophage mannose receptor for effective in vivo imaging of tumor-associated macrophages. Cancer Res. 2012;72:4165-77
47. Ulrich CM. Folate and cancer prevention: a closer look at a complex picture. Am J Clin Nutr. 2007;86:271-3
48. Choi SW, Mason JB. Folate and carcinogenesis: an integrated scheme. J Nutr. 2000;130:129-32
49. Kelemen LE. The role of folate receptor alpha in cancer development, progression and treatment: cause, consequence or innocent bystander? Int J Cancer. 2006;119:243-50
50. Maruti SS, Ulrich CM, White E. Folate and one-carbon metabolism nutrients from supplements and diet in relation to breast cancer risk. Am J Clin Nutr. 2009;89:624-33
51. Ericson U, Borgquist S, Ivarsson MI, Sonestedt E, Gullberg B, Carlson J. et al. Plasma folate concentrations are positively associated with risk of estrogen receptor beta negative breast cancer in a Swedish nested case control study. J Nutr. 2010;140:1661-8
52. Kawai M, Minami Y, Kakizaki M, Kakugawa Y, Nishino Y, Fukao A. et al. Alcohol consumption and breast cancer risk in Japanese women: the Miyagi Cohort study. Breast Cancer Res Treat. 2011;128:817-25
53. Stevens VL, McCullough ML, Sun J, Gapstur SM. Folate and other one-carbon metabolism-related nutrients and risk of postmenopausal breast cancer in the Cancer Prevention Study II Nutrition Cohort. Am J Clin Nutr. 2010;91:1708-15
54. Cheung A, Bax HJ, Josephs DH, Ilieva KM, Pellizzari G, Opzoomer J. et al. Targeting folate receptor alpha for cancer treatment. Oncotarget. 2016;7:52553-74
55. Puig-Kroger A, Sierra-Filardi E, Dominguez-Soto A, Samaniego R, Corcuera MT, Gomez-Aguado F. et al. Folate Receptor beta Is Expressed by Tumor-Associated Macrophages and Constitutes a Marker for M2 Anti-inflammatory/Regulatory Macrophages. Cancer Res. 2009;69:9395-403
56. Chandrupatla DMSH, Molthoff CFM, Lammertsma AA, van der Laken CJ, Jansen G. The folate receptor as a macrophage-mediated imaging and therapeutic target in rheumatoid arthritis. Drug Deliv Transl Res. 2019;9:366-78
57. Jager NA, Westra J, Golestani R, van Dam GM, Low PS, Tio RA. et al. Folate Receptor-beta Imaging Using Tc-99m-Folate to Explore Distribution of Polarized Macrophage Populations in Human Atherosclerotic Plaque. J Nucl Med. 2014;55:1945-51
58. Muller C, Forrer F, Schibli R, Krenning EP, de Jong M. SPECT study of folate receptor-positive malignant and normal tissues in mice using a novel Tc-99m-radiofolate. J Nucl Med. 2008;49:310-7
59. Muller C, Vlahov IR, Santhapuram HKR, Leamon CP, Schibli R. Tumor targeting using Ga-67-DOTA-Bz-folate - investigations of methods to improve the tissue distribution of radiofolates. Nucl Med Biol. 2011;38:715-23
60. Betzel T, Muller C, Groehn V, Muller A, Reber J, Fischer CR. et al. Radiosynthesis and Preclinical Evaluation of 3 '-Aza-2 '-[F-18]fluorofolic Acid: A Novel PET Radiotracer for Folate Receptor Targeting. Bioconjug Chem. 2013;24:205-14
61. Shen J, Putt KS, Visscher DW, Murphy L, Cohen C, Singhal S. et al. Assessment of folate receptor-beta expression in human neoplastic tissues. Oncotarget. 2015;6:14700-9
62. Fabriek BO, van Bruggen R, Deng DM, Ligtenberg AJ, Nazmi K, Schornagel K. et al. The macrophage scavenger receptor CD163 functions as an innate immune sensor for bacteria. Blood. 2009;113:887-92
63. Kristiansen M, Graversen JH, Jacobsen C, Sonne O, Hoffman HJ, Law SK. et al. Identification of the haemoglobin scavenger receptor. Nature. 2001;409:198-201
64. Nguyen TT, Schwartz EJ, West RB, Warnke RA, Arber DA, Natkunam Y. Expression of CD163 (hemoglobin scavenger receptor) in normal tissues, lymphomas, carcinomas, and sarcomas is largely restricted to the monocyte/macrophage lineage. Am J Surg Pathol. 2005;29:617-24
65. Lau SK, Chu PG, Weiss LM. CD163: a specific marker of macrophages in paraffin-embedded tissue samples. Am J Clin Pathol. 2004;122:794-801
66. Fabriek BO, Dijkstra CD, van den Berg TK. The macrophage scavenger receptor CD163. Immunobiology. 2005;210:153-60
67. Eichendorff S, Svendsen P, Bender D, Keiding S, Christensen EI, Deleuran B. et al. Biodistribution and PET imaging of a novel [68Ga]-anti-CD163-antibody conjugate in rats with collagen-induced arthritis and in controls. Mol Imaging Biol. 2015;17:87-93
68. Etzerodt A, Rasmussen MR, Svendsen P, Chalaris A, Schwarz J, Galea I. et al. Structural basis for inflammation-driven shedding of CD163 ectodomain and tumor necrosis factor-alpha in macrophages. J Biol Chem. 2014;289:778-88
69. Flannagan RS, Jaumouille V, Grinstein S. The cell biology of phagocytosis. Annu Rev Pathol. 2012;7:61-98
70. Nahrendorf M, Zhang H, Hembrador S, Panizzi P, Sosnovik DE, Aikawa E. et al. Nanoparticle PET-CT imaging of macrophages in inflammatory atherosclerosis. Circulation. 2008;117:379-87
71. Perez-Medina C, Tang J, Abdel-Atti D, Hogstad B, Merad M, Fisher EA. et al. PET Imaging of Tumor-Associated Macrophages with 89Zr-Labeled High-Density Lipoprotein Nanoparticles. J Nucl Med. 2015;56:1272-7
72. Terry SY, Boerman OC, Gerrits D, Franssen GM, Metselaar JM, Lehmann S. et al. (1)(1)(1)In-anti-F4/80-A3-1 antibody: a novel tracer to image macrophages. Eur J Nucl Med Mol Imaging. 2015;42:1430-8
73. Hamann J, Koning N, Pouwels W, Ulfman LH, van Eijk M, Stacey M. et al. EMR1, the human homolog of F4/80, is an eosinophil-specific receptor. Eur J Immunol. 2007;37:2797-802
74. Rashidian M, Keliher EJ, Bilate AM, Duarte JN, Wojtkiewicz GR, Jacobsen JT. et al. Noninvasive imaging of immune responses. Proc Natl Acad Sci USA. 2015;112:6146-51
75. Park SJ, Kim B, Choi S, Balasubramaniam S, Lee SC, Lee JY. et al. Imaging inflammation using an activated macrophage probe with Slc18b1 as the activation-selective gating target. Nat Commun. 2019;10:1111
76. Huang HJ, Isakow W, Byers DE, Engle JT, Griffin EA, Kemp D. et al. Imaging pulmonary inducible nitric oxide synthase expression with PET. J Nucl Med. 2015;56:76-81
77. Chae SY, Choi CM, Shim TS, Park Y, Park CS, Lee HS. et al. Exploratory Clinical Investigation of (4S)-4-(3-18F-Fluoropropyl)-L-Glutamate PET of Inflammatory and Infectious Lesions. J Nucl Med. 2016;57:67-9
78. Malmberg C, Ripa RS, Johnbeck CB, Knigge U, Langer SW, Mortensen J. et al. 64Cu-DOTATATE for Noninvasive Assessment of Atherosclerosis in Large Arteries and Its Correlation with Risk Factors: Head-to-Head Comparison with 68Ga-DOTATOC in 60 Patients. J Nucl Med. 2015;56:1895-900
79. Li X, Heber D, Leike T, Beitzke D, Lu X, Zhang X. et al. [68Ga]Pentixafor-PET/MRI for the detection of Chemokine receptor 4 expression in atherosclerotic plaques. Eur J Nucl Med Mol Imaging. 2018;45:558-66
80. Verdoes M, Edgington LE, Scheeren FA, Leyva M, Blum G, Weiskopf K. et al. A nonpeptidic cathepsin S activity-based probe for noninvasive optical imaging of tumor-associated macrophages. Chem Biol. 2012;19:619-28
81. Withana NP, Ma X, McGuire HM, Verdoes M, van der Linden WA, Ofori LO. et al. Non-invasive Imaging of Idiopathic Pulmonary Fibrosis Using Cathepsin Protease Probes. Sci Rep. 2016;6:19755
82. Sawa-Wejksza K, Kandefer-Szerszen M. Tumor-Associated Macrophages as Target for Antitumor Therapy. Arch Immunol Ther Exp (Warsz). 2018;66:97-111
83. Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. 2017;14:399-416
84. Bak SP, Walters JJ, Takeya M, Conejo-Garcia JR, Berwin BL. Scavenger receptor-A-targeted leukocyte depletion inhibits peritoneal ovarian tumor progression. Cancer Res. 2007;67:4783-9
85. Nagai T, Tanaka M, Tsuneyoshi Y, Xu B, Michie SA, Hasui K. et al. Targeting tumor-associated macrophages in an experimental glioma model with a recombinant immunotoxin to folate receptor beta. Cancer Immunol Immunother. 2009;58:1577-86
86. Graversen JH, Moestrup SK. Drug Trafficking into Macrophages via the Endocytotic Receptor CD163. Membranes (Basel). 2015;5:228-52
87. Zhan X, Jia L, Niu Y, Qi H, Chen X, Zhang Q. et al. Targeted depletion of tumour-associated macrophages by an alendronate-glucomannan conjugate for cancer immunotherapy. Biomaterials. 2014;35:10046-57
88. Van Gorp H, Delputte PL, Nauwynck HJ. Scavenger receptor CD163, a Jack-of-all-trades and potential target for cell-directed therapy. Mol Immunol. 2010;47:1650-60
89. Kim JS. Combination Radioimmunotherapy Approaches and Quantification of Immuno-PET. Nucl Med Mol Imaging. 2016;50:104-11
90. Kong F, Gao F, Li H, Liu H, Zhang Y, Zheng R. et al. CD47: a potential immunotherapy target for eliminating cancer cells. Clin Transl Oncol. 2016;18:1051-5
91. Gu S, Ni T, Wang J, Liu Y, Fan Q, Wang Y. et al. CD47 Blockade Inhibits Tumor Progression through Promoting Phagocytosis of Tumor Cells by M2 Polarized Macrophages in Endometrial Cancer. J Immunol Res. 2018;2018:6156757
92. Ozpiskin OM, Zhang L, Li JJ. Immune targets in the tumor microenvironment treated by radiotherapy. Theranostics. 2019;9:1215-31
93. Walle T, Martinez Monge R, Cerwenka A, Ajona D, Melero I, Lecanda F. Radiation effects on antitumor immune responses: current perspectives and challenges. Ther Adv Med Oncol. 2018;10:1758834017742575
94. Teresa Pinto A, Laranjeiro Pinto M, Patricia Cardoso A, Monteiro C, Teixeira Pinto M, Filipe Maia A. et al. Ionizing radiation modulates human macrophages towards a pro-inflammatory phenotype preserving their pro-invasive and pro-angiogenic capacities. Sci Rep. 2016;6:18765
95. Genard G, Lucas S, Michiels C. Reprogramming of Tumor-Associated Macrophages with Anticancer Therapies: Radiotherapy versus Chemo- and Immunotherapies. Front Immunol. 2017;8:828
96. Leitha T. Nuclear medicine: proof of principle for targeted drugs in diagnosis and therapy. Curr Pharm Des. 2009;15:173-87
97. Larson SM, Carrasquillo JA, Cheung NK, Press OW. Radioimmunotherapy of human tumours. Nat Rev Cancer. 2015;15:347-60
98. Choi J, Beaino W, Fecek RJ, Fabian KPL, Laymon CM, Kurland BF. et al. Combined VLA-4-Targeted Radionuclide Therapy and Immunotherapy in a Mouse Model of Melanoma. J Nucl Med. 2018;59:1843-9
99. Smith JJ, Sorensen AG, Thrall JH. Biomarkers in imaging: realizing radiology's future. Radiology. 2003;227:633-8
100. Cai W, Chen X. Multimodality molecular imaging of tumor angiogenesis. J Nucl Med. 2008;49(Suppl 2):113S-28S
101. Oyen WJ, van der Graaf WT. Molecular imaging of solid tumors: exploiting the potential. Nat Rev Clin Oncol. 2009;6:609-11
102. Schmitz SA, Taupitz M, Wagner S, Wolf KJ, Beyersdorff D, Hamm B. Magnetic resonance imaging of atherosclerotic plaques using superparamagnetic iron oxide particles. Journal of Magnetic Resonance Imaging. 2001;14:355-61
103. Hildebrandt IJ, Gambhir SS. Molecular imaging applications for immunology. Clin Immunol. 2004;111:210-24
104. Simon GH, von Vopelius-Feldt J, Wendland MF, Fu Y, Piontek G, Schlegel J. et al. MRI of arthritis: comparison of ultrasmall superparamagnetic iron oxide vs. Gd-DTPA. J Magn Reson Imaging. 2006;23:720-7
105. Simon GH, von Vopelius-Feldt J, Fu Y, Schlegel J, Pinotek G, Wendland MF. et al. Ultrasmall supraparamagnetic iron oxide-enhanced magnetic resonance imaging of antigen-induced arthritis: a comparative study between SHU 555 C, ferumoxtran-10, and ferumoxytol. Invest Radiol. 2006;41:45-51
106. Melancon MP, Lu W, Huang QA, Thapa P, Zhou DP, Ng C. et al. Targeted imaging of tumor-associated M2 macrophages using a macromolecular contrast agent PG-Gd-NIR813. Biomaterials. 2010;31:6567-73
107. Locke LW, Mayo MW, Yoo AD, Williams MB, Berr SS. PET imaging of tumor associated macrophages using mannose coated Cu-64 liposomes. Biomaterials. 2012;33:7785-93
108. Leimgruber A, Berger C, Cortez-Retamozo V, Etzrodt M, Newton A, Waterman P. et al. Behavior of Endogenous Tumor-Associated Macrophages Assessed In vivo Using a Functionalized Nanoparticle. Neoplasia. 2009;11:459-U58
109. Jiang C, Cai H, Peng X, Zhang P, Wu X, Tian R. Targeted Imaging of Tumor-Associated Macrophages by Cyanine 7-Labeled Mannose in Xenograft Tumors. Mol Imaging. 2017;16:1536012116689499
110. Lisy MR, Hartung A, Lang C, Schüler D, Richter W, Reichenbach JR. et al. Fluorescent bacterial magnetic nanoparticles as bimodal contrast agents. Invest Radiol. 2007;42:235-41
111. Moore A, Weissleder R, Bogdanov A Jr. Uptake of dextran-coated monocrystalline iron oxides in tumor cells and macrophages. J Magn Reson Imaging. 1997;7:1140-5
112. Yang R, Sarkar S, Yong VW, Dunn JF. In vivo MR Imaging of Tumor-Associated Macrophages: The Next Frontier in Cancer Imaging. Magn Reson Insights. 2018;11:1178623X18771974
113. Tan M, Wu X, Jeong EK, Chen Q, Parker DL, Lu ZR. An effective targeted nanoglobular manganese(II) chelate conjugate for magnetic resonance molecular imaging of tumor extracellular matrix. Mol Pharm. 2010;7:936-43
114. Pan D, Schmieder AH, Wickline SA, Lanza GM. Manganese-based MRI contrast agents: past, present and future. Tetrahedron. 2011;67:8431-44
115. Gohr-Rosenthal S, Schmitt-Willich H, Ebert W, Conrad J. The demonstration of human tumors on nude mice using gadolinium-labelled monoclonal antibodies for magnetic resonance imaging. Invest Radiol. 1993;28:789-95
116. Curtet C, Maton F, Havet T, Slinkin M, Mishra A, Chatal JF. et al. Polylysine-Gd-DTPAn and polylysine-Gd-DOTAn coupled to anti-CEA F(ab')2 fragments as potential immunocontrast agents. Relaxometry, biodistribution, and magnetic resonance imaging in nude mice grafted with human colorectal carcinoma. Invest Radiol. 1998;33:752-61
117. Ayat NR, Qin JC, Cheng H, Roelle S, Gao S, Li Y. et al. Optimization of ZD2 Peptide Targeted Gd(HP-DO3A) for Detection and Risk-Stratification of Prostate Cancer with MRI. ACS Med Chem Lett. 2018;9:730-5
118. Tan M, Wu X, Jeong EK, Chen Q, Lu ZR. Peptide-targeted Nanoglobular Gd-DOTA monoamide conjugates for magnetic resonance cancer molecular imaging. Biomacromolecules. 2010;11:754-61
119. Dekaban GA, Hamilton AM, Fink CA, Au B, de Chickera SN, Ribot EJ. et al. Tracking and evaluation of dendritic cell migration by cellular magnetic resonance imaging. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2013;5:469-83
120. Wermuth PJ, Jimenez SA. Gadolinium Compounds Signaling through TLR 4 and TLR 7 in Normal Human Macrophages: Establishment of a Proinflammatory Phenotype and Implications for the Pathogenesis of Nephrogenic Systemic Fibrosis. J Immunol. 2012;189:318-27
121. Daldrup-Link HE. Ten Things You Might Not Know about Iron Oxide Nanoparticles. Radiology. 2017;284:616-29
122. Umashankar A, Corenblum MJ, Ray S, Valdez M, Yoshimaru ES, Trouard TP. et al. Effects of the iron oxide nanoparticle Molday ION Rhodamine B on the viability and regenerative function of neural stem cells: relevance to clinical translation. Int J Nanomedicine. 2016;11:1731-48
123. Lu M, Cohen MH, Rieves D, Pazdur R. FDA report: Ferumoxytol for intravenous iron therapy in adult patients with chronic kidney disease. Am J Hematol. 2010;85:315-9
124. Toth GB, Varallyay CG, Horvath A, Bashir MR, Choyke PL, Daldrup-Link HE. et al. Current and potential imaging applications of ferumoxytol for magnetic resonance imaging. Kidney Int. 2017;92:47-66
125. Daldrup-Link HE, Golovko D, Ruffell B, Denardo DG, Castaneda R, Ansari C. et al. MRI of tumor-associated macrophages with clinically applicable iron oxide nanoparticles. Clin Cancer Res. 2011;17:5695-704
126. Aghighi M, Theruvath AJ, Pareek A, Pisani LL, Alford R, Muehe AM. et al. Magnetic Resonance Imaging of Tumor-Associated Macrophages: Clinical Translation. Clin Cancer Res. 2018;24:4110-8
127. Simon GH, Bauer J, Saborovski O, Fu YJ, Corot C, Wendland MF. et al. T1 and T2 relaxivity of intracellular and extracellular USPIO at 1.5T and 3T clinical MR scanning. Eur Radiol. 2006;16:738-45
128. Iv M, Samghabadi P, Holdsworth S, Gentles A, Rezaii P, Harsh G. et al. Quantification of Macrophages in High-Grade Gliomas by Using Ferumoxytol-enhanced MRI: A Pilot Study. Radiology. 2019;290:198-206
129. DeNardo D, Andreu P, Coussens LM. Interactions between lymphocytes and myeloid cells regulate pro- versus anti-tumor immunity. Cancer Metastasis Rev. 2010;29:309-16
130. Leek RD, Hunt NC, Landers RJ, Lewis CE, Royds JA, Harris AL. Macrophage infiltration is associated with VEGF and EGFR expression in breast cancer. J Pathol. 2000;190:430-6
131. Mukhtar RA, Moore AP, Nseyo O, Baehner FL, Au A, Moore DH. et al. Elevated PCNA+ tumor-associated macrophages in breast cancer are associated with early recurrence and non-Caucasian ethnicity. Breast Cancer Res Treat. 2011;130:635-44
132. Williams CB, Yeh ES, Soloff AC. Tumor-associated macrophages: unwitting accomplices in breast cancer malignancy. NPJ Breast Cancer. 2016;2:15025
133. Tseng D, Volkmer JP, Willingham SB, Contreras-Trujillo H, Fathman JW, Fernhoff NB. et al. Anti-CD47 antibody-mediated phagocytosis of cancer by macrophages primes an effective antitumor T-cell response. Proc Natl Acad Sci USA. 2013;110:11103-8
134. Mohanty S, Yerneni K, Theruvath JL, Graef CM, Nejadnik H, Lenkov O. et al. Nanoparticle enhanced MRI can monitor macrophage response to CD47 mAb immunotherapy in osteosarcoma. Cell Death Dis. 2019;10:36
135. Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A. et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050-9
136. Fucikova J, Kralikova P, Fialova A, Brtnicky T, Rob L, Bartunkova J. et al. Human tumor cells killed by anthracyclines induce a tumor-specific immune response. Cancer Res. 2011;71:4821-33
137. Chao MP, Jaiswal S, Weissman-Tsukamoto R, Alizadeh AA, Gentles AJ, Volkmer J. et al. Calreticulin Is the Dominant Pro-Phagocytic Signal on Multiple Human Cancers and Is Counterbalanced by CD47. Sci Transl Med. 2010;2:63ra94
138. Nowak AK, Lake RA, Robinson BW. Combined chemoimmunotherapy of solid tumours: improving vaccines? Adv Drug Deliv Rev. 2006;58:975-90
139. Sharkey J, Starkey Lewis PJ, Barrow M, Alwahsh SM, Noble J, Livingstone E. et al. Functionalized superparamagnetic iron oxide nanoparticles provide highly efficient iron-labeling in macrophages for magnetic resonance-based detection in vivo. Cytotherapy. 2017;19:555-69
140. Wu YL, Ye Q, Foley LM, Hitchens TK, Sato K, Williams JB. et al. In situ labeling of immune cells with iron oxide particles: an approach to detect organ rejection by cellular MRI. Proc Natl Acad Sci USA. 2006;103:1852-7
141. Evans ER, Bugga P, Asthana V, Drezek R. Metallic Nanoparticles for Cancer Immunotherapy. Mater Today (Kidlington). 2018;21:673-85
142. Patsialou A, Wyckoff J, Wang YR, Goswami S, Stanley ER, Condeelis JS. Invasion of Human Breast Cancer Cells In vivo Requires Both Paracrine and Autocrine Loops Involving the Colony-Stimulating Factor-1 Receptor. Cancer Res. 2009;69:9498-506
143. Hassan N, Cahill J, Rajasekaran S, Kovey K. Ferumoxytol infusion in pediatric patients with gastrointestinal disorders: first case series. Ann Pharmacother. 2011;45:e63
144. Singh A, Patel T, Hertel J, Bernardo M, Kausz A, Brenner L. Safety of ferumoxytol in patients with anemia and CKD. Am J Kidney Dis. 2008;52:907-15
145. Makela AV, Gaudet JM, Foster PJ. Quantifying tumor associated macrophages in breast cancer: a comparison of iron and fluorine-based MRI cell tracking. Sci Rep. 2017;7:42109
146. Leftin A, Koutcher JA. Quantification of Nanoparticle Enhancement in Polarized Breast Tumor Macrophage Deposits by Spatial Analysis of MRI and Histological Iron Contrast Using Computer Vision. Contrast Media Mol Imaging. 2018;2018:3526438
147. Dadfar SM, Roemhild K, Drude NI, von Stillfried S, Knuchel R, Kiessling F. et al. Iron oxide nanoparticles: Diagnostic, therapeutic and theranostic applications. Adv Drug Deliv Rev. 2019;138:302-25
148. Corot C, Robert P, Idee JM, Port M. Recent advances in iron oxide nanocrystal technology for medical imaging. Adv Drug Deliv Rev. 2006;58:1471-504
149. Klenk C, Gawande R, Uslu L, Khurana A, Qiu D, Quon A. et al. Ionising radiation-free whole-body MRI versus (18)F-fluorodeoxyglucose PET/CT scans for children and young adults with cancer: a prospective, non-randomised, single-centre study. Lancet Oncol. 2014;15:275-85
150. Ansari C, Tikhomirov GA, Hong SH, Falconer RA, Loadman PM, Gill JH. et al. Development of novel tumor-targeted theranostic nanoparticles activated by membrane-type matrix metalloproteinases for combined cancer magnetic resonance imaging and therapy. Small. 2014;10:566-75 417
151. Neuwelt EA, Varallyay CG, Manninger S, Solymosi D, Haluska M, Hunt MA. et al. The potential of ferumoxytol nanoparticle magnetic resonance imaging, perfusion, and angiography in central nervous system malignancy: a pilot study. Neurosurgery. 2007;60:601-11 discussion 11-2
152. Chen L, Ma X, Dang M, Dong H, Hu H, Su X. et al. Simultaneous T Cell Activation and Macrophage Polarization to Promote Potent Tumor Suppression by Iron Oxide-Embedded Large-Pore Mesoporous Organosilica Core-Shell Nanospheres. Adv Healthc Mater. 2019;8:e1900039
153. Reichel D, Tripathi M, Perez JM. Biological Effects of Nanoparticles on Macrophage Polarization in the Tumor Microenvironment. Nanotheranostics. 2019;3:66-88
154. Shen Z, Song J, Yung BC, Zhou Z, Wu A, Chen X. Emerging Strategies of Cancer Therapy Based on Ferroptosis. Adv Mater. 2018;30:e1704007
155. Landry R, Jacobs PM, Davis R, Shenouda M, Bolton WK. Pharmacokinetic study of ferumoxytol: a new iron replacement therapy in normal subjects and hemodialysis patients. Am J Nephrol. 2005;25:400-10
156. Jung M, Weigert A, Mertens C, Rehwald C, Brune B. Iron Handling in Tumor-Associated Macrophages-Is There a New Role for Lipocalin-2? Front Immunol. 2017;8:1171
157. Zanganeh S, Hutter G, Spitler R, Lenkov O, Mahmoudi M, Shaw A. et al. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat Nanotechnol. 2016;11:986-94
158. Laskar A, Eilertsen J, Li W, Yuan XM. SPION primes THP1 derived M2 macrophages towards M1-like macrophages. Biochem Biophys Res Commun. 2013;441:737-42
159. Sindrilaru A, Peters T, Wieschalka S, Baican C, Baican A, Peter H. et al. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J Clin Invest. 2011;121:985-97
160. Tan HY, Wang N, Li S, Hong M, Wang X, Feng Y. The Reactive Oxygen Species in Macrophage Polarization: Reflecting Its Dual Role in Progression and Treatment of Human Diseases. Oxid Med Cell Longev. 2016;2016:2795090
161. Gray A, de la Luz Garcia-Hernandez M, van West M, Kanodia S, Hubby B, Kast WM. Prostate cancer immunotherapy yields superior long-term survival in TRAMP mice when administered at an early stage of carcinogenesis prior to the establishment of tumor-associated immunosuppression at later stages. Vaccine. 2009;27(Suppl 6):G52-9
162. Apostolopoulos V, Pietersz GA, Tsibanis A, Tsikkinis A, Drakaki H, Loveland BE. et al. Pilot phase III immunotherapy study in early-stage breast cancer patients using oxidized mannan-MUC1 [ISRCTN71711835]. Breast Cancer Res. 2006;8:R27
163. Matsumoto M, Takeda Y, Tatematsu M, Seya T. Toll-Like Receptor 3 Signal in Dendritic Cells Benefits Cancer Immunotherapy. Front Immunol. 2017;8:1897
164. Zhao J, Zhang Z, Xue Y, Wang G, Cheng Y, Pan Y. et al. Anti-tumor macrophages activated by ferumoxytol combined or surface-functionalized with the TLR3 agonist poly (I: C) promote melanoma regression. Theranostics. 2018;8:6307-21
165. Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE. et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060-72
166. Kim SE, Zhang L, Ma K, Riegman M, Chen F, Ingold I. et al. Ultrasmall nanoparticles induce ferroptosis in nutrient-deprived cancer cells and suppress tumour growth. Nat Nanotechnol. 2016;11:977-85
167. Zhou Z, Song J, Tian R, Yang Z, Yu G, Lin L. et al. Activatable Singlet Oxygen Generation from Lipid Hydroperoxide Nanoparticles for Cancer Therapy. Angew Chem Int Ed Engl. 2017;56:6492-6
168. Zhang C, Bu W, Ni D, Zhang S, Li Q, Yao Z. et al. Synthesis of Iron Nanometallic Glasses and Their Application in Cancer Therapy by a Localized Fenton Reaction. Angew Chem Int Ed Engl. 2016;55:2101-6
169. Huang KJ, Wei YH, Chiu YC, Wu SR, Shieh DB. Assessment of zero-valent iron-based nanotherapeutics for ferroptosis induction and resensitization strategy in cancer cells. Biomater Sci. 2019;7:1311-22
170. Wang YX, Idee JM. A comprehensive literatures update of clinical researches of superparamagnetic resonance iron oxide nanoparticles for magnetic resonance imaging. Quant Imaging Med Surg. 2017;7:88-122
171. Hao D, Ai T, Goerner F, Hu X, Runge VM, Tweedle M. MRI contrast agents: basic chemistry and safety. J Magn Reson Imaging. 2012;36:1060-71
172. Costa da Silva M, Breckwoldt MO, Vinchi F, Correia MP, Stojanovic A, Thielmann CM. et al. Iron Induces Anti-tumor Activity in Tumor-Associated Macrophages. Front Immunol. 2017;8:1479
173. Kodali V, Littke MH, Tilton SC, Teeguarden JG, Shi L, Frevert CW. et al. Dysregulation of macrophage activation profiles by engineered nanoparticles. ACS Nano. 2013;7:6997-7010
174. Rojas JM, Sanz-Ortega L, Mulens-Arias V, Gutierrez L, Perez-Yague S, Barber DF. Superparamagnetic iron oxide nanoparticle uptake alters M2 macrophage phenotype, iron metabolism, migration and invasion. Nanomedicine. 2016;12:1127-38
175. Mulens-Arias V, Rojas JM, Perez-Yague S, Morales MP, Barber DF. Polyethylenimine-coated SPIONs trigger macrophage activation through TLR-4 signaling and ROS production and modulate podosome dynamics. Biomaterials. 2015;52:494-506
176. Golovin YI, Gribanovsky SL, Golovin DY, Klyachko NL, Majouga AG, Master capital A C. et al. Towards nanomedicines of the future: Remote magneto-mechanical actuation of nanomedicines by alternating magnetic fields. J Control Release. 2015;219:43-60
177. Kang H, Kim S, Wong DSH, Jung HJ, Lin S, Zou K. et al. Remote Manipulation of Ligand Nano-Oscillations Regulates Adhesion and Polarization of Macrophages in vivo. Nano Lett. 2017;17:6415-27
178. Toraya-Brown S, Sheen MR, Zhang P, Chen L, Baird JR, Demidenko E. et al. Local hyperthermia treatment of tumors induces CD8(+) T cell-mediated resistance against distal and secondary tumors. Nanomedicine. 2014;10:1273-85
179. Janko C, Ratschker T, Nguyen K, Zschiesche L, Tietze R, Lyer S. et al. Functionalized Superparamagnetic Iron Oxide Nanoparticles (SPIONs) as Platform for the Targeted Multimodal Tumor Therapy. Front Oncol. 2019;9:59
180. Mühlberger MCJ, Unterweger H, Schreiber E, Band J, Lehmann C. et al. Functionalization of T lymphocytes for magnetically controlled immune therapy: Selection of suitable superparamagnetic iron oxide nanoparticles. J Magn Magn Mater. 2019;473:61-7
181. Perica K, Bieler JG, Schutz C, Varela JC, Douglass J, Skora A. et al. Enrichment and Expansion with Nanoscale Artificial Antigen Presenting Cells for Adoptive Immunotherapy. ACS Nano. 2015;9:6861-71
182. Zhang Q, Wei W, Wang P, Zuo L, Li F, Xu J. et al. Biomimetic Magnetosomes as Versatile Artificial Antigen-Presenting Cells to Potentiate T-Cell-Based Anticancer Therapy. ACS Nano. 2017;11:10724-32
183. Cho NH, Cheong TC, Min JH, Wu JH, Lee SJ, Kim D. et al. A multifunctional core-shell nanoparticle for dendritic cell-based cancer immunotherapy. Nat Nanotechnol. 2011;6:675-82
184. Zhao Y, Zhao X, Cheng Y, Guo X, Yuan W. Iron Oxide Nanoparticles-Based Vaccine Delivery for Cancer Treatment. Mol Pharm. 2018;15:1791-9
185. Shevtsov MA, Nikolaev BP, Yakovleva LY, Parr MA, Marchenko YY, Eliseev I. et al. 70-kDa heat shock protein coated magnetic nanocarriers as a nanovaccine for induction of anti-tumor immune response in experimental glioma. J Control Release. 2015;220:329-40
186. Mohanty S, Aghighi M, Yerneni K, Theruvath JL, Daldrup-Link H. Improving the efficacy of osteosarcoma therapy: Combining drugs that turn cancer cell “don't eat me” signal off and “eat me” signal on. Molecular Oncology. 2019;13(10):2049-206
Author Biography
 Sudip Mukherjee, Ph.D., AMRSC is working as a postdoctoral research associate at the department of bio-engineering in Rice University, USA. His research is involved in the development of advanced nano/bio materials for (a) drug delivery in cancer theranostics, (b) immunomodulatory applications in cancer & diabetes and (c) angiogenesis. Dr. Mukherjee has published over 36 peer-reviewed research articles, 3 book chapters and 3 patents granted or pending. He serves as international advisory board member of 'Materials Research Express', IOP Sciences. He is an associate member (AMRSC) of Royal Society of Chemistry, UK. Dr. Mukherjee also works as a scientific blog writer for RSC Biomaterials Science. Additionally, he serves as repeated scientific reviewers for several international journals including Chemical Communication, J Mater Chem A, J Mater Chem B, RSC Advances, Biofabrication, Nanotechnology, Journal of Biomedical Nanotechnology, Acta Biomaterialia, Cancers etc. Dr. Mukherjee has received several awards including travel grant from Houston Methodist-Rice University, best poster awards from ICNANO & ICANN, best publication award from CSIR-IICT and young scientist award from KVRAO Scientific society, Hyderabad.
Sudip Mukherjee, Ph.D., AMRSC is working as a postdoctoral research associate at the department of bio-engineering in Rice University, USA. His research is involved in the development of advanced nano/bio materials for (a) drug delivery in cancer theranostics, (b) immunomodulatory applications in cancer & diabetes and (c) angiogenesis. Dr. Mukherjee has published over 36 peer-reviewed research articles, 3 book chapters and 3 patents granted or pending. He serves as international advisory board member of 'Materials Research Express', IOP Sciences. He is an associate member (AMRSC) of Royal Society of Chemistry, UK. Dr. Mukherjee also works as a scientific blog writer for RSC Biomaterials Science. Additionally, he serves as repeated scientific reviewers for several international journals including Chemical Communication, J Mater Chem A, J Mater Chem B, RSC Advances, Biofabrication, Nanotechnology, Journal of Biomedical Nanotechnology, Acta Biomaterialia, Cancers etc. Dr. Mukherjee has received several awards including travel grant from Houston Methodist-Rice University, best poster awards from ICNANO & ICANN, best publication award from CSIR-IICT and young scientist award from KVRAO Scientific society, Hyderabad.
 Dominik Sonanini, MD, is resident in oncology at the Department of Medicine, University of Tübingen and holds a postdoc position in the Werner Siemens Imaging Center under the leadership of Prof. Bernd Pichler, University of Tübingen. His research focuses on the development of novel immunotracers to guide antibody-based and cellular immunotherapies by positron emission tomography. Dr. Sonanini aims to bridge the gap between preclinical tracer development and clinical application of promising molecular imaging approaches.
Dominik Sonanini, MD, is resident in oncology at the Department of Medicine, University of Tübingen and holds a postdoc position in the Werner Siemens Imaging Center under the leadership of Prof. Bernd Pichler, University of Tübingen. His research focuses on the development of novel immunotracers to guide antibody-based and cellular immunotherapies by positron emission tomography. Dr. Sonanini aims to bridge the gap between preclinical tracer development and clinical application of promising molecular imaging approaches.
 Dr. Andreas Maurer, Ph.D. is a research group leader in imaging probe development at the Werner Siemens Imaging Center of the Eberhard Karls University of Tübingen. His research focuses on the development of new imaging probes to explore the functional role of the targeted biomolecules in physiological and pathological pathways. Dr. Maurer is especially interested in cellular stress pathways and the consequences of proteolytic processing and other posttranslational protein modifications on immunity. The broad and interdisciplinary expertise and excellent infrastructure within his group gives the chance to employ state-of-the-art organic synthesis, radiolabeling and imaging techniques to synthesize and characterize new molecular probes. He has published over 25 peer reviewed articles. He has won many awards and given many invited talks. He is a member of Europian society of molecular imaging (ESMI).
Dr. Andreas Maurer, Ph.D. is a research group leader in imaging probe development at the Werner Siemens Imaging Center of the Eberhard Karls University of Tübingen. His research focuses on the development of new imaging probes to explore the functional role of the targeted biomolecules in physiological and pathological pathways. Dr. Maurer is especially interested in cellular stress pathways and the consequences of proteolytic processing and other posttranslational protein modifications on immunity. The broad and interdisciplinary expertise and excellent infrastructure within his group gives the chance to employ state-of-the-art organic synthesis, radiolabeling and imaging techniques to synthesize and characterize new molecular probes. He has published over 25 peer reviewed articles. He has won many awards and given many invited talks. He is a member of Europian society of molecular imaging (ESMI).
 Heike E. Daldrup-Link, MD, PhD, is a physician-scientist and tenured professor at Stanford University. She currently serves as Director of the Pediatric Molecular Imaging Program, Co-Director of the Cancer Imaging Program and Associate Chair for Diversity in the Department of Radiology at Stanford. Her research group focuses on linking the fields of nanotechnology and medical imaging to create more accurate and efficient diagnoses and image-guided therapies. Dr. Daldrup-Link has both uncovered nano-science principles in an NIH funded-basic science lab and brought the most promising innovations to her patients' bedside. Dr. Daldrup-Link has published over 150 peer-reviewed research articles, 20 book chapters and editorials, four edited books, and six patents pending or granted. She serves as an editorial board member for Nanotheranostics and the Journal of Nuclear Medicine and as an internal advisory board member for The Lancet Hematology. Dr. Daldrup-Link received 33 awards for her research work and she is an elected member of the American Society for Clinical Investigation (ASCI, honor society for clinician-scientists) and the American Institute of Medical and Biological Engineering (AIMBE).
Heike E. Daldrup-Link, MD, PhD, is a physician-scientist and tenured professor at Stanford University. She currently serves as Director of the Pediatric Molecular Imaging Program, Co-Director of the Cancer Imaging Program and Associate Chair for Diversity in the Department of Radiology at Stanford. Her research group focuses on linking the fields of nanotechnology and medical imaging to create more accurate and efficient diagnoses and image-guided therapies. Dr. Daldrup-Link has both uncovered nano-science principles in an NIH funded-basic science lab and brought the most promising innovations to her patients' bedside. Dr. Daldrup-Link has published over 150 peer-reviewed research articles, 20 book chapters and editorials, four edited books, and six patents pending or granted. She serves as an editorial board member for Nanotheranostics and the Journal of Nuclear Medicine and as an internal advisory board member for The Lancet Hematology. Dr. Daldrup-Link received 33 awards for her research work and she is an elected member of the American Society for Clinical Investigation (ASCI, honor society for clinician-scientists) and the American Institute of Medical and Biological Engineering (AIMBE).
![]() Corresponding author: Prof. Heike Daldrup-Link <heikededu>
Corresponding author: Prof. Heike Daldrup-Link <heikededu>
 Global reach, higher impact
Global reach, higher impact