13.3
Impact Factor
Theranostics 2019; 9(23):6824-6839. doi:10.7150/thno.36739 This issue Cite
Review
PSMA-targeting agents for radio- and fluorescence-guided prostate cancer surgery
1. Department of Radiology and Nuclear Medicine, Radboud University Medical Center, Nijmegen, The Netherlands
2. Radboud University Nijmegen, Institute for Molecules and Materials, Systems Chemistry, Nijmegen, The Netherlands
3. Department of Urology, Radboud University Medical Center, Nijmegen, The Netherlands
4. Department of Nuclear Medicine, University Hospital Bonn, Bonn, Germany
Received 2019-5-16; Accepted 2019-7-16; Published 2019-9-20
Abstract
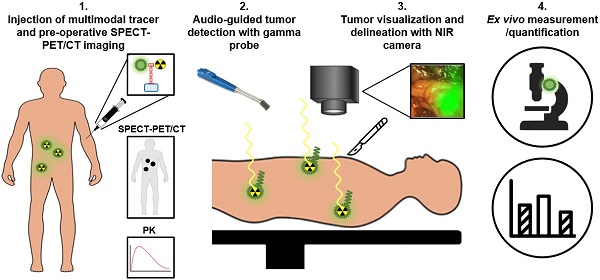
Despite recent improvements in imaging and therapy, prostate cancer (PCa) still causes substantial morbidity and mortality. In surgical treatment, incomplete resection of PCa and understaging of possible undetected metastases may lead to disease recurrence and consequently poor patient outcome. To increase the chance of accurate staging and subsequently complete removal of all cancerous tissue, prostate specific membrane antigen (PSMA) targeting agents may provide the surgeon an aid for the intraoperative detection and resection of PCa lesions. Two modalities suitable for this purpose are radionuclide detection, which allows sensitive intraoperative localization of tumor lesions with a gamma probe, and fluorescence imaging, allowing tumor visualization and delineation. Next to fluorescence, use of photosensitizers may enable intraoperative targeted photodynamic therapy to eradicate remaining tumor lesions. Since radiodetection and optical imaging techniques each have their own strengths and weaknesses, a combination of both modalities could be of additional value. Here, we provide an overview of recent preclinical and clinical advances in PSMA-targeted radio- and fluorescence-guided surgery of PCa.
Keywords: PSMA, image-guided surgery, radionuclide, fluorescence, multimodal imaging.
Introduction
Despite recent improvements in imaging and therapy, PCa remains the most frequently diagnosed cancer type in men and is estimated to be the second leading cause of cancer related deaths [1, 2]. Currently, PCa treatment is dependent on the stage of the disease at initial diagnosis. Possible early stage management consists of active surveillance or watchful waiting. However, if treatment is deemed necessary, due to progression of the disease under active surveillance, or if the initial stage of the disease mandates immediate treatment, curative options are indicated. One of these curative options is surgical removal of all cancerous tissue: radical prostatectomy with or without pelvic lymph node dissection [3]. Depending on the extent of the disease, surgery may be combined with adjuvant radiotherapy, hormone therapy and chemotherapy, especially in patients with high risk PCa, lymph node positive disease or positive surgical margins after resection [3].
Surgical treatment of PCa faces two main challenges. First, complete resection of the prostate tumor, and thus managing negative surgical margins, remains difficult [4]. While removal of the entire prostate gland makes complete resection of the primary tumor feasible, it often leads to nerve damage that may cause debilitating functional side effects such as urinary incontinence and erectile dysfunction. Therefore, surgeons are often on the proverbial knife edge between complete oncological resection and nerve-saving operations with a maximum chance of good functional outcome, but a higher chance of positive resection margins as a consequence [4]. The average rate of positive surgical margins after radical prostatectomy is 15% and can increase up to 50% in men with more locally advanced disease [4, 5]. Hence, margin detection is of utmost importance for the surgical management and clinical outcomes of PCa patients. The second main challenge is the detection of tumor positive lymph nodes visualized in preoperative 68Ga/18F-PSMA PET or nano-MRI scans [4, 6, 7]. The ability to find and resect metastatic lymph node tissue is currently based on anatomical landing sites like the obturator region together with the external and internal ileac artery region. Most small nodes are invisible for the surgeons eye, and are not palpable during surgery [7]. Intraoperative tissue shifts, atypical locations, small size and inconspicuous morphology hamper the detection of tumor positive lymph nodes during surgery and thus their resection [6, 7]. In addition, metastatic tumor lesions can be difficult to resect due to proximity to other tissue (nerves, blood vessels, bladder and rectum) [4]. The effect of incomplete resection can be profound for the individual patient as it may lead to early disease recurrence and poor patient outcome [8, 9]. To increase the chance of complete resection of all tumor tissue, more sensitive and specific techniques are needed that allow for intraoperative real time detection of all cancer tissue including the tiniest lesions.
In order to detect PCa, prostate-specific membrane antigen (PSMA), a type II transmembrane glycoprotein, represents an excellent target for several reasons. Firstly, PSMA is over-expressed on >90% of all primary PCa lesions, as well as tumor positive lymph nodes and distant metastasis. In contrast, PSMA expression on healthy tissues is minimal [10-12]. Secondly, PSMA expression in PCa correlates with the Gleason grading of the prostate lesions, with high expressions in high Gleason scores [13]. Finally, binding of a targeting agent to the active center of the extracellular domain of PSMA generally leads to internalization of the agent, resulting in enhanced retention of the label in the lesion [6]. Due to these properties, PSMA constitutes a reliable molecular marker for PCa and is an ideal target for imaging and therapy of PCa. So far, a wide variety of PSMA-targeting agents have been described, varying from intact antibodies to low-molecular-weight compounds [14]. Small molecules exploit the binding of substrates, including N-acetyl-L-aspartyl-L-glutamate (NAAG) and folate (poly)gamma glutamate substrates, to the active site of PSMA [15, 16]. First, glutamate containing phosphoramidates or 2-(phosphinylmethyl) pentanedioic acid based PSMA-binding motifs were synthesized [17, 18]. Later, glutamate-urea-lysine based PSMA-binding motifs were developed and are now most frequently used [19]. The latter compounds are structurally more similar to NAAG and therefore exhibit the best PSMA-binding properties [20].
Pre- and intraoperative identification of malignant tissue could be improved using multiple techniques including radioguidance, fluorescence imaging, ultrasound- and/or MRI-guidance. In this review we will focus on and discuss the potential of the currently available PSMA-targeting agents for radio-, fluorescence- and multimodal-guided intraoperative detection of PCa lesions. In addition, the feasibility of near infrared (NIR) photosensitizer-conjugated PSMA-tracers for intraoperative targeted photodynamic therapy (tPDT) is discussed.
PSMA-radioguided surgery in PCa
Over the past five years, the intraoperative PSMA-radioguided surgery (RGS) approach entered the operating theater for PCa patients undergoing prostatectomy with or without extended pelvic lymphadenectomy. Radioguided lymph node dissections were applied in an experimental setting in patients both within the typical field of an extended lymph node template (iliaca externa, interna, communis, obturator fossa) and patients with atypically localized lesions (retrovesical/seminal, presacral/pararectal, retroperitoneal) [21-23]. RGS is based on the detection of γ-photons with handheld probes. These γ-photons have virtually infinite penetration through human tissue. Consequently, intraoperative detection of radionuclides is generally not hindered by penetration depth and is considered highly sensitive [24]. The above mentioned properties of RGS make this technique highly suitable to tackle the second challenge in PCa surgery; incomplete detection of tumor positive lymph nodes. Despite the high sensitivity, the intraoperative use of radionuclides remains limited as surgeons need to rely on an acoustic signal and exact visualization of the tumor tissue is lacking, which may cause inaccurate tumor delineation [24]. Moreover, due to the high penetration depth of background signals from tracers accumulating in other organs accurate detection can be hampered. For instance, detection in the vicinity of the kidneys is impossible for tracers with high kidney retention, the same goes for the bladder [6]. Intraoperative radionuclide imaging using a handheld SPECT camera is currently not commonly used as an imaging method, as its value is limited by a low spatial resolution, lack of anatomical reference and relatively high radiation exposure [14].
PSMA-based SPECT/CT and PET/CT can accurately detect primary PCa and small lymph node metastasis up to a size of 0.5-1 cm3 preoperatively [6]. However, during surgery, reliable identification of small and/or atypically localized lesions can be challenging which can lead to incomplete resection of lymph node metastasis or extensive removal of non-affected lymph nodes. Recent studies investigated the precision of salvage lymph node dissection (sLND), a still controversial surgical approach for PCa patients [25-27]. In this sLND approach the dissection field and removal of lymph nodes is based on well-defined surgical regions in combination with advanced imaging such as preoperative 68Ga/18F-PSMA-PET imaging. Among patients that undergo sLND a biochemical response is reported in 40-60% of the patients and a complete biochemical response is only observed in 20-30% of the patients. One of the reasons for these disappointing results could be the incomplete surgical resections of all involved tumor positive lymph nodes [25, 27]. In another study, final pathology revealed no metastases in lesions removed based on preoperative PET/CT imaging in 31% of the patients with conventional surgery, despite the removal of on average 13.6 (range: 5-27) lymph nodes per patient [26]. Hence, the surgical treatment for patients with PCa needs improvement. This might be achieved by combining preoperative radionuclide imaging with intraoperative radionuclide detection. For example, many resections involve significant tissue movements and deformation, making preoperative scan-based navigation imprecise [6]. For the surgeon, the combined knowledge from the preoperative scans and real-time feedback during RGS might therefore provide vital information on the location, the extend of the disease and positive surgical margins. Moreover, it may aid the surgeon in minimizing the invasiveness of the surgical procedure [28].
In PCa surgery, radioguided resection using 99mTc-nanocolloid was first introduced for sentinel lymph node dissections. After injection in the prostate, the 99mTc-nanocolloid distributed to lymph nodes along the internal and external iliac vessels and the obturator fossa [29]. Even though this technique does not use a PSMA-targeted tracer, it shows the potential of radionuclide-guided resection [30, 31]. The effectiveness of radioguided sentinel lymph node dissections was tested in a large study in 2,000 patients by Holl et al. [29]. They reported a detection rate of 98%. Nonetheless, in 24% of the cases, not all preoperatively visualized nodes could be removed during surgery, presumably due to nodal uptake as low as 0.07% injected dose, leading to a count rate too low for intraoperative detection of the node [32, 33]. Of the entire number of SLN (n=12141, in 2020 patients) resected only 600 (4.9%) were positive for metastases, indicating the need for more specific cancer detection strategies in radioguided resection of PCa and its metastasis [29]. Nonetheless, accuracy of sentinel lymph node procedures in PCa could be improved, for instance with the use of a hybrid indocyanine green-99mTc-nanocolloid (radioactive and fluorescent) [31].
With the introduction of PSMA-targeted RGS (PSMA-RGS) detection of both primary and distant PSMA-positive tumors was made possible [6]. PSMA-RGS makes use of radiolabeled PSMA-targeting agents such as antibodies, peptides, and small molecules [21, 23, 34-36]. Use of PSMA-targeting tracers for RGS potentially allows the detection of tiny and atypically located lesions [6]. In a PSMA-RGS feasibility study, Maurer et al. used the [111In]In-DOTAGA-(3-iodo-y)-f-k-Sub(KuE) (111In-PSMA-I&T, Figure 1) small molecule ligand to evaluate PSMA-RGS for the detection of metastatic PCa lesions [35]. Five patients received an intravenous injection of 124 MBq 111In-PSMA-I&T, 24 hours before surgery. Metastatic tissue was tracked intraoperatively using a gamma probe and all tissues that showed radiosignal were removed. The presence of tumor tissue was confirmed by ex vivo histopathology.
Chemical structures of [111In]In-DOTAGA-(3-iodo-y)-f-k-Sub(KuE) (PSMA-I&T) and [99mTc]Tc-mas3-y-nal-k(Sub-KuE) (PSMA-I&S).
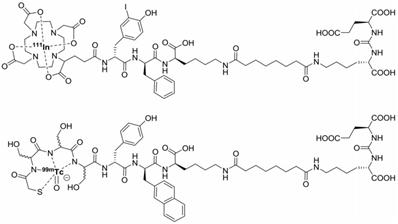
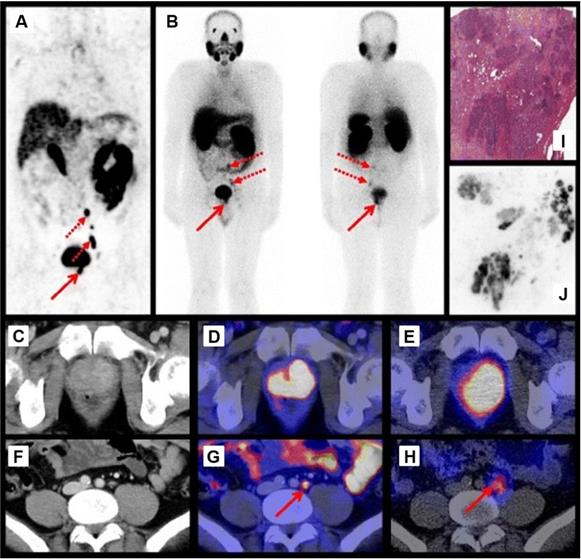
Maurer et al. demonstrated that 111In-PSMA-I&T based RGS allowed the resection of subcentimeter lesions and led to the removal of additional PCa lesions. In 2 patients positive lesions, as confirmed by histopathological analysis, were found that did not show up during preoperative [68Ga]Ga-HBED-CC-PSMA (68Ga-PSMA-11) PET/CT imaging [35]. Schottelius et al. also successfully applied the 111In-PSMA-I&T ligand for radioguided resection of PSMA-positive lesions in an exemplary PCa patient (Figure 2A-J) [22]. Both studies demonstrate the potential of 111In-PSMA-I&T ligand for intraoperative detection of tumor positive lymph nodes in PCa. More recently, Robu et al. explored the potential of [99mTc]Tc-mas3-y-nal-k(Sub-KuE) (99mTc-PSMA-I&S, Figure 1) mediated PSMA-RGS in two exemplary PCa patients [21]. As the half-life of 99mTc is much shorter than that of 111In (6 hours vs. 2.8 days), timing of the surgery is crucial. However, the low energy gamma rays produced by 99mTc could be advantageous for RGS, as gamma probes have the highest sensitivity for predominantly lower energy gamma photon emitting radionuclides [28]. In all suspected lesions previously identified by 68Ga-PSMA-11 PET/CT imaging, a high uptake of 99mTc-PSMA-I&S was observed 12 hours after injection, as measured by preoperative SPECT/CT imaging. This high uptake resulted in successful intraoperative detection and resection of radiosignal-positive lesions [21]. In a retrospective analysis Maurer et al. compared the radioactive rating (positive vs. negative) of tissues resected during 99mTc-PSMA-I&S-RGS to their postoperative histopathological analysis [23]. Tissue specimens were considered positive if a count rate of at least twice the background was measured. The radioactive rating yielded an accuracy of 93% (confidence interval [CI]: 85.5-96.7%), a sensitivity of 83.6% (CI: 70.9-91.5%), and a specificity of 100%. During the 99mTc-PSMA-I&S-RGS all tumor lesions visualized on 68Ga-PSMA-11 PET could be resected and additional small metastasis, down to 3 mm in size, could be removed [23]. In all patients a urinary catheter was inserted that allowed for removal of radioactive urine from the bladder, which otherwise could impair gamma probe measurements. In approximately one third of the patients treated with salvage PSMA-RGS, surgery related complications were noted, mostly consisting of a (temporary) worsening of incontinence, bladder leakage or lymphedema, which also occur during conventional surgery [23, 24, 36].
Preoperative imaging using 68Ga-PSMA-11 PET/CT 1 h p.i. (A) and 111In-PSMA-I&T SPECT/CT and planar scintigraphy (4 h p.i., 155 MBq) (B). Axial 68Ga-PSMA-11 PET/CT images of the primary tumor in the prostate (D) and a representative lymph node (G). Corresponding CT images (C, F) and axial 111In-PSMA-I&T SPECT/CT images (E, H). H&E staining (I) and 111In-autoradiography (J) of cryosections from resected prostate tissue. The human study was approved by the institutional review boards of the participating medical institutions, and the patient provided signed informed consent. Reprinted with permission from Schottelius et al., 111In-PSMA-I&T: expanding the spectrum of PSMA-I&T applications towards SPECT and radioguided surgery, Copyright 2015, Springer [22].
Subsequent to the above mentioned studies multiple clinical follow up studies were performed using either 111In-PSMA-I&T or 99mTc-PSMA-I&S mediated PSMA-RGS. In these studies PSA levels and time to biochemical recurrence were monitored [23, 24, 36]. However, no comparison was made to the conventional surgical treatment of PCa. So far, no randomized trials have been performed that compare PSMA-RGS with a conventional surgical approach, in which the dissection field is based solely on preoperative 68Ga/18F-PSMA-PET imaging. However, designing these studies is challenging as surgical fields are difficult to define, outcomes of surgeries are hard to compare due to inter-surgeon quality differences, and the long survival of the patients hampers fast oncological outcome measurements. Nonetheless, larger patient numbers and long-term follow-up studies are needed to determine the clinical benefit of PSMA-RGS. These studies should include comparison of treatment-related complications, progression-free survival, overall survival and quality of life of PCa patients.
Taken together, PSMA-RGS still represents an individual and not guideline-conform treatment concept without proven benefit on quality of life or cancer-specific survival, despite the large quantities of proof of principle data [37]. Nevertheless, PSMA-RGS is feasible and is an aid for the surgeon to achieve complete resection of all PCa lesions using audio-based intraoperative and ex vivo gamma-probe measurements. Ultimately, this might lead to improved oncological outcomes. Still, use of radioguidance could only solve one of the two major challenges in PCa surgery. Even though it might improve detection and removal of tumor positive lymph nodes, it is not an optimal aid for the surgeon in visualization and precise delineation of the primary tumor and hard-to-resect metastasis.
PSMA-targeted intraoperative NIRF imaging
In recent years, an alternative intraoperative imaging method, near infrared fluorescence (NIRF) imaging, has entered the surgical theatre. Fluorescence imaging might be used to tackle the first challenge in the surgical treatment of PCa: achieving negative surgical resection margins [38]. It relies on fluorescent markers to accumulate in malignant tissue and produce optical photons upon absorption of light of a specific wavelength, which can in turn be detected by a fluorescence camera system [39]. Normal tissue exhibits very low auto-fluorescence in the NIR spectrum, resulting in high signal to background ratios. With NIR imaging techniques, tumor lesions can be directly visualized and clearly distinguished from healthy tissue [39, 40]. Other advantages of fluorophores are that they can be excited repeatedly, have extended half-lives and have a high spatial and temporal resolution [38]. However, the vast majority of the photons produced by NIR fluorophores will be scattered or absorbed within a few cell layers of tissue [40]. This means that fluorescence imaging is limited by low tissue penetration depth [41]. Another essential challenge is the lipophilicity of many NIR fluorophores, which may significantly change the pharmacokinetics and tumor targeting properties of the targeting ligands [42].
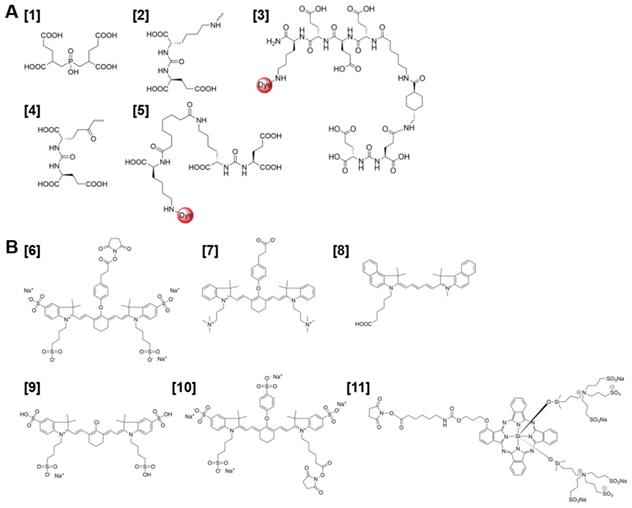
PSMA-targeted NIRF imaging is emerging as an attractive strategy for visual guidance during PCa surgery. The success of this strategy largely depends on the production of PSMA-targeted NIRF probes with optimal in vivo targeting characteristics. A series of PSMA-targeted contrast agents have been developed by conjugating different NIR fluorophores to PSMA-targeting whole antibodies, antibody fragments and small molecules [34, 43, 44]. The first small molecule PSMA-NIR fluorophore conjugates were produced by Humblet et al. who conjugated the highly potent PSMA inhibitor GPI [1] to IRDye78 [6] to create the molecule GPI-78 (Figure 3A-B). Interestingly, the NIR fluorophore conjugation enhanced PSMA binding affinity over 20-fold. According to the authors this was due to the four sulfonic acid groups present in the IRDye78 that also show PSMA binding affinity [43]. This study emphasizes a main challenges of using small PSMA binding motifs for NIR-dye conjugation; varieties in imaging moieties and their linkers can lead to major differences in affinity, pharmacokinetics and tumor uptake of the tracer. This does not necessarily have to be a disadvantage. For example, an increase in the length of the linker was described to increase affinity for PSMA and the addition of multiple negative charges showed improved tumor-to-background ratios [45-48]. Bao et al. systematically conjugated different NIR fluorophores to the glutamate-urea-lysine PSMA binding motif [2] [34]. The PSMA binding motif coupled to the ZW800+3C fluorophore [7], with a linker length of 18 Å and a net charge of minus 6, showed optimal in vitro and in vivo PSMA binding. In addition to the effects of the linker, the physicochemical properties of conjugated fluorophores can also drastically alter characteristics of the small PSMA-targeting molecules. Changes were observed not only in hydrophobicity and polarity but also in binding affinity [34, 43, 44]. Wang et al. synthesized a high-affinity PSMA ligand called PSMA-1 [3] and labeled it with NIR dyes IRDye800 and Cy5.5 [8]. These PSMA-1-NIR probes were shown to selectively target orthotopic PSMA-positive PC3-PIP tumors and allowed intraoperative tumor visualization using NIRF-imaging. Their results indicated that the pharmacokinetics of the probes were highly dependent on the type of fluorophore conjugated to the PSMA-1 ligand. These differences in pharmacokinetics were observed in blood clearance, tumor retention, liver and kidney uptake. Overall, the most hydrophilic and negatively charged dyes cleared fastest [44]. Another property that should be taken into account is the lipophilicity of a fluorophore, which affects binding properties and internalization of the tracer and thereby its retention in the tumor [49].
Despite the fact that attachment of NIR dyes could alter small molecule PSMA-binding properties, NIR-conjugated ligands already showed high potential in multiple preclinical studies. However, it should be noted that the in vivo tumor targeting properties of the different NIR-conjugated ligands cannot be directly compared, as PSMA expression levels differ among the various tumor models used. For example, uptake of [177Lu]Lu-DOTA-PSMA-617 tracer varied between 8 %ID/g and 44 % ID/g for LNCaP and PC3-PIP xenografts respectively [48, 50]. Kularatne et al. developed a NIR tracer using a small molecule PSMA inhibitor called DUPA [4] and the S0456 NIR dye [9] [51]. This tracer was shown to avidly bind PSMA positive tumors with high specificity in mice. The fluorescent signal in PSMA-positive tumors lasted for more than 48 hours, allowing visualization throughout fluorescence-guided surgery [51]. Moreover, Kelderhouse et al. developed PSMA-specific probes by combining fluorophores like Alexafluor 647, Dylight680 and IRDye800CW [10] with the PSMA inhibitor DUPA [4] [52]. Tracers were tested in a metastatic mouse model. Intravenous injection of these PSMA-ligand NIR dye tracers enabled fluorescence imaging of the PSMA-expressing metastases, allowing their complete resection with minimal contamination of surrounding healthy tissues. The intraoperative resection of these tumor lesions was performed in stages, meaning that larger lesions were resected first. Importantly, excision of these more prominent fluorescent masses often revealed smaller lesions that could have been missed without the aid of PSMA-specific fluorescence (Figure 4A-D) [52]. Another low-molecular weight urea-based fluorescent PSMA binding agent called YC-27 [5] was conjugated to the NIR dye IRDye800CW [10].
Kovar et al. described that YC-27-800CW showed good tissue contrast and tumor delineation at doses as low as 0.25 nmol [1]. Subsequently, Neuman et al. used a PCa murine model with subcutaneous PC3-PIP PSMA-expressing tumors to perform image-guided resection of tumor lesions [53]. In 8 mice tumors were resected with the guidance of a NIRF imaging system, while the remaining animals (n = 10) were resected under normal white light. None of the animals resected with YC-27 fluorescence-guidance developed a recurrence, whereas 40% of the mice with conventional white light resection developed recurrence within 30 days after resection [53]. Together, these studies suggest that PSMA-binding small molecules or antibody fragments coupled to NIR dyes have the potential to be further developed for clinical use as contrast agents in fluorescence-guided surgery. However, the fact that coupling of the NIR dye can drastically alter the properties of the contrast agent should be taken into account during development of such a tracer. Nevertheless, it is difficult to predict the in vivo properties of these ligands consisting of multiple structural parts that often differ between tracers.
(A) Chemical structure of PSMA-binding motifs GPI [1], glutamate-urea-lysine (KuE) [2], PSMA-1 [3], DUPA [4], and YC27 [5]. (B) Chemical structures of fluorescent dyes IRDye78 [6], ZW800+3C [7], Cy5.5 [8], SO456 [9], IRDye800CW [10], IRDye700DX [11].
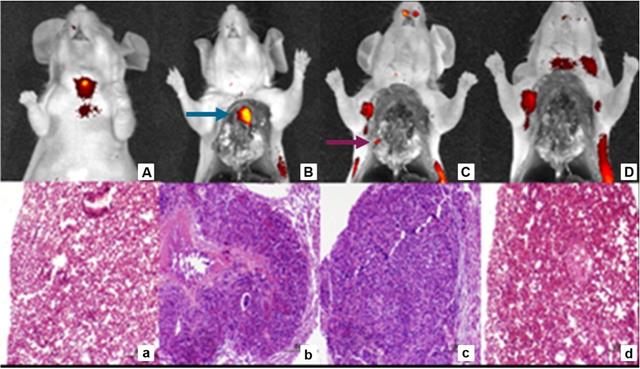
Sequential tumor debulking surgery and H&E analysis of 22RV1 tumor metastases 4 h p.i. with DUPA-IRDye800CW (10 nmol). Fluorescent and white light image overlays of whole body image (A), opened chest cavity (B), after the removal of the primary tumor (blue arrow) (C) and after the removal of all secondary nodules (purple arrow) (D). H&E staining healthy control lung (a), primary tumor (b), secondary tumor nodule (c) and residual tissue (d). Reprinted with permission from Kelderhouse et al., Development of tumor-targeted near infrared probes for fluorescence guided surgery, Copyright 2013, American Chemical Society [52].
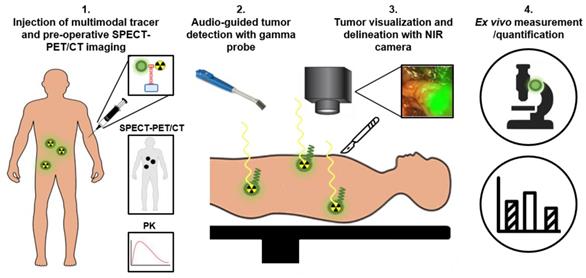
Schematic representation of multimodal radio- and fluorescence-guided surgery. PK: pharmacokinetics
Best of both worlds: PSMA-targeted multimodal image-guided surgery
To overcome the specific drawbacks of the radionuclide and fluorescence-imaging modalities, the focus of intraoperative PSMA-targeted tracer research has shifted towards combining both fluorescence- and radionuclide detection techniques for PCa surgery [5, 54-56]. In case of PCa, multimodal PSMA-targeting agents potentially can be used for four applications (Figure 5). First, the radionuclide allows preoperative PET/CT or SPECT/CT imaging to localize all tumor tissue. Second, a handheld gamma probe can be used to localize both deep-seated and superficial metastatic tumor lesions intraoperatively based on the radiosignal, guiding the surgeon until the fluorescent signal becomes visible. Due to its high penetration depth, radioguidance is especially suitable for the detection and guidance towards metastatic lesions, nonetheless no accurate real time visualization can be made. Therefore, third, the fluorescent label can be used to directly visualize the tumors during surgery, opening up the opportunity for resections without positive surgical margins. Fourth and final, an accurate quantitative assessment can be made to determine the pharmacokinetics of the tracer, to facilitate dose finding studies, and to perform ex vivo examination of resected tissue [14]. By placing both radionuclide and fluorescent tags on the same tracer, potential complications associated with co-injected mixtures can be avoided, including receptor saturation due to limited receptor expression and differences in pharmacokinetics, biodistribution, or PSMA binding affinity [56].
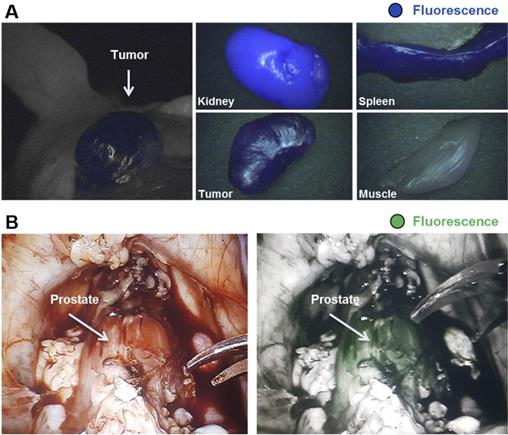
Proof-of-principle fluorescence-guided surgery studies with multimodal 68Ga-IRDye800CW-PSMA-11 in tumor-bearing mice and healthy pigs. (A) 68Ga-IRDye800CW-PSMA-11 (0.5 nmol) was injected in s.c. LNCaP tumor-bearing mice for small-animal PET imaging, followed by ex vivo fluorescence detection 2 h p.i. (IMAGE1 S system). (B) After preimaging acquisition of background fluorescence (da Vinci FireFly system), IRDye800CW-PSMA-11 (30 μg/kg) was injected i.v. in healthy pigs. 1 h p.i. fluorescence-guided prostatectomy using in vivo and ex vivo fluorescence detection was performed. This research was originally published in JNM, Baranski et al., PSMA-11-Derived Dual-Labeled PSMA Inhibitors for Preoperative PET Imaging and Precise Fluorescence-Guided Surgery of Prostate Cancer, J Nucl Med, 2017, © SNMMI [54].
Encouraging results were reported in several preclinical studies that have focused on the development of dual-labeled radionuclide and fluorescent PSMA-targeting agents. Lütje et al. evaluated the potential of multimodal image-guided surgery of PCa with the anti-PSMA monoclonal antibody D2B, labeled with both 111In and the NIR dye IRDye800CW (Figure 3B, [10]) [5]. Two days after injection of the [111In]In-DTPA-D2B-IRDye800CW conjugate, intraperitoneal LS174T PSMA-expressing tumors could be visualized specifically with both imaging modalities. Subsequent image-guided resection showed the feasibility of complete multimodal-guided resection of all intraperitoneal tumor lesions in vivo. Interestingly, in a mouse with several LS174T-PSMA tumors located at different depths in the peritoneal cavity, the limited penetration depth of NIRF imaging was demonstrated. Only the two most superficial tumors could be visualized with NIRF imaging, whereas microSPECT/CT imaging showed several additional tumor lesions [5]. However, the mAb D2B is a murine IgG and should at least be humanized before clinical translation is feasible, which is not the case for dual-labeled small molecule PSMA-ligands. Other advantages of using small-molecule multimodal tracers are fast tumor targeting and rapid blood clearance [11, 57].
In 2011, Banerjee et al. developed a dual-labeled, glutamate-urea based, PSMA-targeting SPECT/NIRF imaging small molecule tracer conjugated with IRDye800CW and a DOTA chelator, labeled with 111In [58]. They showed clear multimodal tumor visualization and high tracer uptake in subcutaneous PSMA-expressing PC3-PIP tumors (16.4±3.7 % ID/g), compared to the PSMA-negative PC3-flu control tumors (1.9± 0.2 % ID/g, 5 hours p.i.). Subcutaneous xenografts were delineated specifically, both with SPECT and NIRF imaging. However, intense tracer uptake was observed in the kidneys, which can be explained by the route of excretion of both tracers [58]. Baranski et al. developed a series of PSMA-11 targeting ligands conjugated with different fluorophores, among which the NIR IRDye800CW and Dylight800 [54]. PSMA-specific uptake of the IRDye800CW (13.6 ± 3.7 %ID/g) and DyLight800 (15.6 ± 5.5 %ID/g) conjugated ligands in the tumor was significantly higher than that of the unconjugated 68Ga-PSMA-11 agent (4.8 ± 1.3 %ID/g). Furthermore, proof of concept fluorescence-guided surgery studies with 68Ga-IRDye800CW-PSMA-11were performed in healthy pigs using a DaVinci robotic surgery system. A PSMA-specific fluorescent signal (1 hour p.i. of the tracer) was observed in the prostate, which expresses PSMA on a physiological level. This demonstrated the potential of the 68Ga-IRDye800CW-PSMA-11 ligands for fluorescence-guided radical prostatectomy (Figure 6A-B) [54].
Overview of PSMA ligands for intraoperative PCa detection
| Reference | Year | PSMA ligand | Radio-nuclide | Fluoro-phore* | Research status | Main results | ||
|---|---|---|---|---|---|---|---|---|
| Radioguided surgery | ||||||||
| Maurer et al. [35] | 2015 | lysine-urea-glutamate | 111In (γ) | - | Clinical feasibility, 5 patients | - Identification of additional positive lesions not detected during preoperative PET/CT imaging | ||
| Schottelius et al. [22] | 2015 | lysine-urea-glutamate | 111In (γ) | - | Exemplary patient | - Radioguided resection of PSMA-positive lesions | ||
| Robu et al. [21] | 2017 | lysine-urea-glutamate | 99mTc (γ) | - | Two exemplary patients | - Successful detection and resection of radiosignal-positive lesions | ||
| Maurer et al. [23] | 2018 | lysine-urea-glutamate | 99mTc (γ) | - | Retrospective analysis, 31 patients | - Comparison of radioactive rating with histopathological analysis; specificity 93%, sensitivity 83.6% | ||
| Rauscher et al. [36] | 2017 | lysine-urea-glutamate | 111In (γ) + 99mTc (γ) | - | Clinical follow up, 55 patients | - PSA level reduction > 50% in 44 (80%), > 90% in 29 (53%) patients | ||
| Horn et al. [24] | 2017 | lysine-urea-glutamate | 111In (γ) + 99mTc (γ) | - | Clinical follow up, 59 patients | - PSA < 0.2 ng/mL in 67% of patients | ||
| Fluorescence-guided surgery | ||||||||
| Humblet et al. [43] | 2005 | GPI | - | IRDye78 (771-796 nm) | Preclinical, s.c. LNCaP | - NIR fluorophore conjugation improved PSMA-affinity over 20-fold | ||
| Wang et al. [44] | 2014 | PSMA-1 | - | IRDye800CW (778-794 nm) + Cy5.5 (675-694 nm) | Preclinical, orthotopic PC3-PIP | - Pharmacokinetics highly dependent on the conjugated fluorophore | ||
| Bao et al. [34] | 2017 | lysine-urea-glutamate | - | Cy5.5 (675-694 nm), Cy7 (753-775 nm) + ZW800+3C (774-789 nm) | Preclinical, s.c. LNCaP | - Physicochemical properties of fluorophores drastically alter characteristics of ligands | ||
| Kularatne et al. [51] | 2018 | DUPA | - | S0456 (788-800 nm) | Preclinical, s.c./orthotopic 22Rv1/LNCaP | - Sub-nanomolar concentration sufficient to visualize small lesions. - Retention of fluorescent signal in PSMA+ tumors > 48 hours | ||
| Kelderhouse et al. [52] | 2013 | DUPA | - | AF647 (650-665 nm), Dylight680 (692-712 nm) + IRDye-800CW (778-794 nm) | Preclinical, intracardial injections 22Rv1 | - Complete resection of metastasis with minimal contamination from healthy tissue | ||
| Kovar et al. [1] | 2014 | YC-27 | - | IRDye-800CW (778-794 nm) | Preclinical, s.c. 22Rv1 | - High tissue contrast and sufficient tumor delineation at doses as low as 0.25 nmol | ||
| Neuman et al. [53] | 2015 | YC-27 | - | IRDye-800CW (778-794 nm) | Preclinical, s.c. PC3-PIP | - 0% recurrences when resected with NIRF-guidance compared to 40% in control white-light mice | ||
| Multimodal-guided surgery | ||||||||
| Banerjee et al.[58] | 2011 | lysine-urea-glutamate | 111In (γ) | IRDye-800CW (778-794 nm) | Preclinical, s.c. PC3-PIP | - Multimodal visualization, delineation and high uptake of the tracer, 16.4±3.7 %ID/g** | ||
| Baranski et al. [54] | 2017 | PSMA-11 | 68Ga (β+) | IRDye-800CW (778-794 nm)+ Dylight800 (777-794 nm) | Preclinical, s.c. LNCaP + healthy pigs | - Conjugation of IRDye800CW (13.6 ± 3.7 %ID/g) and DyLight800 (15.6 ± 5.5 %ID/g) increased specific tumor uptake** - Ligands enabled radical prostatectomy under fluorescence-guidance in pigs | ||
| Schottelius et al. [59] | 2018 | lysine-urea-glutamate | 68Ga (β+) | Sulfo‐Cy5 (646-662 nm) | Precinical, s.c. LNCaP | - PSMA-specific uptake in tumor (4.5 ± 1.8 %ID/g)** - Tumor/background ratios at 1 h p.i. of 2.1 and 9.6 for blood and muscle. | ||
* Fluorescent wavelengths are indicated as excitation maximum-emission maximum in nm.** Note that PSMA expression levels differ between tumor models used. Therefore, uptake values (%ID/g) cannot be directly compared. Abbreviations: p.i.; post injection, s.c.; subcutaneous, PSMA; Prostate specific membrane antigen, h; hours, %ID/g; Percentage injected dose per gram, NIRF; Near-infrared fluorescence.
Very recently, Schottelius et al. developed a tracer named PSMA Imaging and Fluorescence (PSMA-I&F). Based on the previous PSMA-I&T, with addition of the fluorophore Sulfo‐Cy5. PSMA-specific tracer uptake into LNCaP xenografts (4.5 ± 1.8 %ID/g) led to sufficient imaging contrast in 68Ga-PSMA-I&F PET and in intraoperative fluorescence imaging [59]. However, in both studies mentioned above, only the fluorescent signal was used for guidance during resection, providing the first proof of concept for multimodal image-guided resection. For future work, it would be interesting to use both detection modalities during surgery, as for instance has been done in renal cell carcinoma [60], to gain insight into the added value of combining both imaging modalities.
So far, these preclinical studies demonstrate the potential value of multimodal strategies for image guidance during surgery. However, translation of the PSMA-targeting multimodal preclinical tracers to the clinical setting still has to be made. For other malignancies, multimodal tracers were translated to the clinic, indicating the potential of multimodal treatments in patients. For example, Hekman et al. showed that tumor targeted multimodal imaging using [111In]In-DOTA-girentuximab-IRDye800CW is safe and can be used for intraoperative guidance in patients with clear cell renal cell carcinoma [60]. Nonetheless, additional studies are needed to evaluate the additional value of multimodal image-guided surgery compared to conventional mono-modal surgery techniques for complications, quality of life and overall survival and patient outcome.
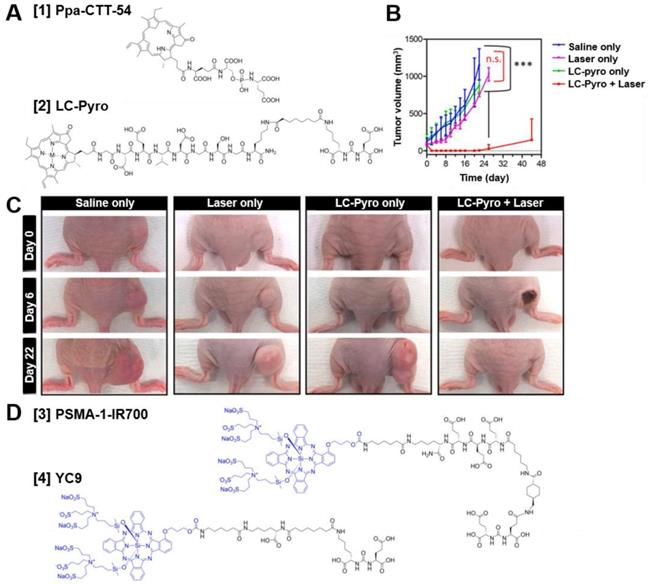
Chemical structures of different PSMA-targeted photosensitizer conjugates and example photodynamic therapy (PDT) efficacy of LC-Pyro in vivo. (A) Chemical structures of Ppa-CTT-54 [1] and LC-Pyro [2]. (B) PDT efficacy of LC-Pyro in PSMA+ PC3-PIP s.c. tumor-bearing mice. Tumor growth curves (mean ±SD, n = 4 for each group, ***P ≤ 0.001, n.s. = not significant). (C) Representative images of tumor-burdened mice in saline only, laser only, LC-Pyro only, and LC-Pyro + Laser groups at 0, 6, and 22 days post-PDT treatment. (D) Chemical structures of PSMA-1-IR700 [3] and YC9 [4]. Reprinted and adapted with permission from Harmatys et al., Tuning pharmacokinetics to improve tumor accumulation of a prostate-specific membrane antigen-targeted phototheranostic agent, Copyright 2018, American Chemical Society [64].
Theranostics: Photodynamic therapy using photosensitizer-conjugated PSMA tracers
In some cases, achieving complete resection of tumor tissue is challenging. For example when lymph nodes or positive tumor margins are located in close proximity to surrounding healthy tissue (e.g. nerves, bladder) [4]. These difficult to resect tumor lesions can potentially be eradicated by targeted photodynamic therapy (tPDT) [57]. The three components needed for tPDT are a light, oxygen and a photosensitizer. Upon activation, the photosensitizer undergoes an oxygen mediated photochemical process producing reactive oxygen species (ROS) which results in specific cellular damage of the target cells [61]. In addition, tPDT may even lead to systemic immunity due to destruction of tumor cells inducing an anti-tumor immune response [57]. As PSMA-targeted tracers with a photosensitizer are designed to accumulate in PCa lesions and the light (normal or laparoscopic 680nm laser) can be focused to the tumor site as well, tPDT is highly precise. Potentially, it enables therapy with minimal side effects [57]. The first PSMA-targeted photosensitizer conjugates consisted of small molecule PSMA inhibitors coupled to porphyrin dyes. With these conjugates, including Ppa-CTT-54 [1] and LC-Pyro [2] (Figure 7A) feasibility of porphyrin-mediated PSMA-targeted PDT was shown in vivo (Figure 7B-C) [61-64]. Further analysis showed caspase pathway activation, cleavage of polyADP-ribose polymerase (PARP), DNA fragmentation and rapid cytoskeletal disruption, leading to apoptosis and/or necrosis [61]. Next to the porphyrin dyes, most studies have focused on small molecule PSMA inhibitors coupled to the photosensitizer IRDye700DX (690nm), including the tracers called PSMA-1-IR700 [3] and YC9 [4] (Figure 7D) [65, 66]. In vivo light irradiation using these PSMA-IRDye700DX tracers led to significant tumor size reduction of PC3-PIP PSMA-positive s.c. tumors compared to the PSMA-negative tumors, without apparent off-target toxicity. Moreover, a delay in tumor growth and an increase in mean survival were observed, demonstrating the potential to effectively inhibit PC3-PIP tumor progression with these tracers. Next, Watanabe et al. conjugated IRDye700DX to the humanized J591 anti-PSMA antibody and its fragments [67]. They showed significant tumor growth delay and prolonged survival in mice bearing PSMA-positive PC3 tumors, again indicating the feasibility of IRDye700DX mediated tPDT. The first study to describe tPDT with multimodal IRDye700DX tracers for PCa detection, resection and irradiation was performed by Lütje et al [68]. They developed the theranostic PSMA targeting agent [111In]In-DTPA-D2B-IRDye700DX by conjugating DTPA and IRDye700DX (Figure 3B, [11]) to the murine anti-PSMA antibody D2B and subsequently labeled it with 111In. In 5 mice, intraoperative NIRF imaging could be used for guided resection of subcutaneous LS174T-PSMA tumor xenografts, demonstrating the applicability of the multimodal conjugate for intraoperative image-guided resection of tumor lesions. tPDT treatment of LS174T-PSMA tumor bearing mice using this tracer caused significant tumor growth delay, as tumors in the control group reached a size of 500 mm3 in 27.7 days (range: 19-42 days), while treated tumors reached this size after 50.4 days (range: 23-80 days). In addition, median survival of the treated mice (47 days) was significantly longer than that of the untreated mice (27days). This study provided the first proof-of-principle that multimodal [111In]In-DTPA-D2B-IRDye700DX can be used for pre- and intraoperative detection of PSMA positive tumors with radionuclide- and fluorescence-imaging, surgical guidance, and PSMA-targeted PDT. PSMA-targeted PDT for PCa therapy is still in its infancy, no clinical proof of concept studies have been performed yet. However, despite the fact that a lot of translational steps towards the clinic still need to be taken, PSMA-targeted PDT has a high potential to become a valuable therapeutic option especially to remove remaining tumor cells in PCa patients during and after surgery.
Discussion
In order to image PCa cells via PSMA-expression a suitable tracer is required. Important requirements for this are: 1) High uptake in PSMA-positive tumor tissue, 2) good tumor retention up to the moment of surgery and 3) low uptake in background tissue. Sufficient tumor-to-background ratios are key for targeting and imaging of prostate cancer. Given the variety of available and clinically used PSMA tracers, it is difficult to reach consensus on which ligand, radionuclide and/or fluorophore combination is best suited for a particular application. Still, PSMA-targeting ligands should be designed to meet a specific clinical need. In case of intraoperative PCa detection several factors are important as will be discussed below.
Type of targeting agent
A lot of effort has been put into finding the optimal PSMA-binding small molecule, antibody fragment or whole antibody. Important properties of these PSMA-binding tracers are specificity for PSMA and sufficient accumulation and retention in the tumor to allow intraoperative detection. The first described PSMA binding tracers are monoclonal antibodies (mAbs) [69]. Well known high affinity anti-PSMA mAbs include the J415, J533 and J591 series, which bind to the extracellular domain of PSMA [69, 70]. These mAbs hold promise for PCa detection and therapy. The main advantage of mAbs is the high absolute uptake in the tumor, caused by their high affinity and long circulatory half-life. In addition, compared to the renal clearance of small molecules, the hepatic clearance of mAbs leads to less background signal in the surgical field of PCa. However, because of their large size, accumulation in solid tumors can be slow (taking up to days) and high non-specific uptake in PSMA-negative tumors is observed, presumably as a result of the EPR effect [67]. Despite the long circulating plasma half-lives, high uptake in normal tissue, and low tumor-to-normal tissue ratios, the high tumor uptake of these mAbs could be essential in NIRF imaging and tPDT, where a sufficiently high amount of tracer in the tumor is essential for imaging sensitivity and therapeutic outcomes [11, 71].
To overcome the disadvantages of mAbs and their derivates, small molecule PSMA-binding motifs with improved pharmacokinetics were developed [11, 72]. The small size of the tracers could improve penetration of the dense tumor tissue, which is advantageous from both the diagnostic and the therapeutic point of view [57]. The most commonly used small molecule PSMA tracers have glutamate-urea-lysine based PSMA-binding motifs [19, 72]. They are structurally most similar to the normal PSMA substrate (NAAG) and therefore exhibit the best PSMA-binding properties [20]. Despite the above mentioned advantages of small molecules, the use of small molecule PSMA binding motifs also poses several challenges. First, high background signal from the kidneys and bladder, due to the renal clearance of the small molecules, might hamper accurate intraoperative detection of PCa in the vicinity of these organs. Second, small molecules may have a lower absolute tumor uptake (in terms of %ID/g) when compared to mAbs, which could lead to concentrations that are too low to detect, visualize and treat with radiodetection, NIRF imaging and tPDT, respectively. Last, addition of imaging moieties and linkers can lead to major differences in affinity, pharmacokinetics and tumor uptake of the tracer. Nonetheless, multiple recent studies have shown that these challenges can be overcome and that, by creating NIRF/multimodality probes, the binding properties and pharmacokinetics of small molecule PSMA-ligands can even be improved [34, 54, 59]. In general, poor vascularization and heterogeneity of the disease could impede PSMA-targeting of both mAbs and small molecules for surgical guidance and treatment, leading to false negative detection rates. Nonetheless, PSMA is highly expressed on more than >90% of all lesions [12]. Next to PSMA-targeting agents, other tracers not discussed in the current review have the potential to improve the surgical treatment of PCa. These include for example folate or androgen receptor targeting agents, sodium fluoride (NaF) tracers and amino acid (mostly leucine) analogs [73, 74].
Type of radionuclide
In addition to choosing the optimal tracer for PSMA targeted intraoperative detection of PCa, it is of utmost importance to choose the most suitable imaging moieties. The most commonly used radionuclides for intraoperative gamma probe based PCa detection are 99mTc and 111In, which have several main advantages. First, besides intraoperative detection, these γ-emitters can be used for pre- and post-operative SPECT imaging. Second, 99mTc and 111In have half-lives of 6 hours and 2.8 days respectively, leading to sufficient time for preoperative SPECT scanning, multiple-hour and/or next day surgeries. The 6-hour half-life of 99mTc is better compatible with the fast pharmacokinetics of small molecule PSMA-tracers and could therefore be preferred over 111In. Last, the availability of the radionuclides is important. 99mTc is readily available in many hospitals, leading to a high availability even in regional hospitals [21]. In addition to its availability, 99mTc often is preferred over 111In because of its lower production costs, and shorter half-life, leading to lower radiation exposure to both patients and nuclear medicine personnel. Moreover, 99mTc emits lower energy gamma rays of 140 keV, that are preferred for collimation, and a greater activity can be administered per patient resulting in higher photon yields and better quality SPECT images [21, 75, 76].
Beside low-energy gamma emitting radionuclides (e.g. 99mTc and 111In), RGS could also be performed using positron emitters such as 68Ga or 18F [77]. However, for the detection of high-energy 511 keV gamma emissions a dedicated gamma-detection probe is needed with thicker shielding and/or a longer collimator [28], which results in drastically increased dimensions and overall weight of the probe. This probe will generally not fit through a standard trocar used during laparoscopic and robot-assisted surgery. As the current surgical treatment of patients with PCa is shifting towards a minimally-invasive setting, use of positron-emitting isotopes for RGS might therefore be limited [28, 38]. Moreover, a recent study by Orsaria et al. reported poor performance of [18F]-FDG gamma probe-guided surgery in evaluating axillary lymph nodes in breast cancer patients, because of high background gamma levels from local and distant parts of the body [78].
Type of fluorophore
In general, dyes that have emission wavelengths in the near-infrared (NIR) region of the spectrum (650-900 nm) are employed for intraoperative imaging. Main advantages of using these NIR dyes are a superior tissue penetration depth, reduced interference from the presence of blood in the surgical field and low auto-fluorescence of the tissue in the NIR region [47]. Indocyanine green (ICG, emission peak at 830 nm) is one of the few fluorophores approved for intraoperative clinical use by the Food and Drug Administration (FDA). However, ICG by itself is non-target specific and in oncology mostly used to map tumor-draining lymph nodes [79]. A number of other NIR dyes have shown great promise in preclinical in vivo imaging studies, including Cy5.5, Cy7, Dylight800, ICG derivatives and IRDye800CW. In a study to determine which of these dyes shows the best in vivo properties, all dyes were labeled to the same PSMA-binding small molecule. Both the Cy7- and IRDye800CW-ligands showed superior PSMA-specific tumor uptake, internalization and tumor-to-background ratios. Moreover, the IRDye800CW-ligands displayed a much higher fluorescent intensity [47]. In another head-to-head comparison of different fluorescent dyes, IRDye800CW and IRDye700DX were found highly suitable for fluorescence imaging due to their brightness and photostability [80]. The IRDye800CW is often preferred over IRDye700DX for imaging purposes, as it is higher in the NIR spectrum, has a higher intensity and is a smaller and more stable molecule under in vivo conditions. Nonetheless, IRDye700DX has the major advantage of being a potent photosensitizer. This means that, during surgery, theranostic IRDye700DX tracers can also be used to eradicate the remaining unresectable cancer cells using tPDT [81]. Hence, different dyes with varying characteristics are suitable for intraoperative imaging and the exact choice of dye will also highly depend upon the on the specifications of the camera used for detection in the operating room.
PSMA-guidance for PCa detection in minimally invasive surgery
With the rapid growth of minimally-invasive laparoscopic and/or robot-assisted PCa surgery, there is a need for image-guidance technologies suitable for these procedures. One important development in fluorescence-guided robotic surgery is the introduction of the Intuitive Surgical's Firefly™ imaging technology (Novadaq Technologies, Mississauga, ON, Canada) integrated in the da Vinci® robot.[53, 82] This surgical system is equipped with filters that are optimized for detection of indocyanine green (ICG, peak emission wavelength 830 nm), but could also be used for the detection of IRDye800CW (peak emission wavelength 792 nm). For detection of this IRDye800CW, proof of concept has been demonstrated with the PSMA-ligand YC-27 [53]. However, no filters for the detection of fluorescent dyes in other spectra are available yet. This hampers the use of photosensitizer-conjugated theranostic PSMA-ligands (peak emission wavelength ~650-700 nm) in minimally invasive fluorescence-guided surgery [64, 65].
In RGS, laparoscopic gamma probes are used for in vivo and ex vivo measurement of the radiosignal in minimally-invasive surgical procedures. However, these probes have limited maneuverability in vivo and therefore hamper the detection of low activity lesions that are located near high background areas such as the kidneys [83]. To overcome this problem, van Oosterom et al. have developed a DROP-IN gamma probe with an effective scanning direction range between 0-180°, that was proven to be a valuable tool for robot-assisted RGS procedures [83, 84].
Opportunity for PSMA-guided surgery in other malignancies
PSMA is expressed on the neovascular endothelium of numerous non-prostate solid tumors [85-88]. Chang et al. studied PSMA-expression in the neovasculature of 15 non-prostate tumors using five different PSMA mAbs. In this study a strong neovascular PSMA immunoreactivity was described in many tumors, including clear cell renal cell carcinoma, colonic adenocarcinoma, glioblastoma multiforme, non-small cell lung carcinoma and breast carcinoma [86]. Targeting PSMA-expression in the tumor associated neovascular endothelium could therefore enable intra-operative imaging of a variety of malignancies. Despite the fact that intra-operative PSMA-imaging studies of these other malignancies were not described in literature yet, successful PSMA-based pre- and post-operative imaging support the idea that targeted intra-operative PSMA-imaging, to prevent positive surgical margins, could be extended beyond PCa. For instance, in a pilot study by Sasikumar et al. the feasibility of pre- and postoperative imaging of gliomas using 68Ga-PSMA-11 PET/CT was investigated [89]. From the 10 patients scanned preoperatively, 9 were positive and proven to be a true recurrence in post-surgical pathological analysis. In 3 patients scanned immediately after surgery, the presence or absence of disease could be identified on the 68Ga-PSMA-11 scan and correlated with conventional MRI imaging. Furthermore, in a recent study by Meyer et al. the added value of pre-operative PSMA-based 18F-DCFPyL PET/CT imaging was suggested in patients with oligometastatic renal cell carcinoma [90]. In 28.6% of the patients a total of 12 more lesions were identified on 18F-DCFPyL PET/CT compared to conventional imaging, leading to detection rates of 88.9% and 66.7%, respectively.
However, in other cancer types absolute tumor uptake of the PSMA-tracers is lower compared to PCa. Expression of PSMA is only present on the neovasculature, with little to no expression on the tumor cells and the normal vascular endothelium, possibly leading to lower detection rates. Therefore, PSMA-based imaging is most suitable for tumors with a high rate of neovascularization.
Conclusion and Future Perspective
The detection and removal of positive resection margins, small tumor lesions and micro-metastasis still poses major challenges in the surgical management of patients with PCa. Unfortunately, at the moment surgeons have to choose between complete oncological resection, which often leads to debilitating side effects, and nerve-saving operations focused on functional outcomes, with a higher chance of positive resection margins as a consequence [9]. With the development of highly specific PSMA ligands, intraoperative PCa detection and the essential margin detection becomes available. Given the variety of the developed PSMA tracers, together with the fact that no consensus has been found on which tracer is most suitable for intraoperative use, in this manuscript we discussed considerations for the design of such PSMA tracers.
It is debatable which type of tracer is best suited for intra operative use, as both mAbs and small molecule PSMA tracers each have their own advantages, including high tumor uptake of mAbs and high specificity and fast pharmacokinetics of small molecule PSMA tracers. Modifications in small molecule PSMA-tracers, including linker length and addition of multiple negative charges, were found to alter the binding affinity of the PSMA ligands causing improved targeting characteristics and reduced background signals. At present, 99mTc and 111In-labeled small molecule PSMA ligands, already used in a clinical studies, seem to be the most promising tracers for radioguided surgery. Currently, for PCa surgical removal of cancerous tissue is performed without any fluorescence image guidance. In the up-and-coming fluorescence-guided surgery field, NIR dye labeled PSMA ligands show encouraging results. In addition, photosensitizers like IRDye700DX could be used for tPDT of residual tumor lesions. Hence, use of such a theranostic dual-labeled PSMA ligand allows for imaging, resection and local killing of PCa cells.
Presumably the most promising developments in the intraoperative field are the dual-labeling strategies that allow for both acoustic and visual detection of PSMA-expressing tumor lesions, micro-metastases and positive resection margins. Together, these multimodal strategies empower guided total resection of PCa tumors. Unfortunately, so far, fluorescent and dual-labeled PSMA-targeting tracers have only been investigated in a preclinical setting. In order to move the field forward, it is essential that proper prospective clinical studies are carried out measuring the additional value of multimodal image guided surgery compared to conventional surgery techniques. Clinical trials comparing these two treatments should be performed and main outcomes of these studies should include comparison of toxicology, complications, progression free survival, overall survival and quality of life of the PCa patients.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Kovar JL, Cheung LL, Simpson MA, Olive DM. Pharmacokinetic and Biodistribution Assessment of a Near Infrared-Labeled PSMA-Specific Small Molecule in Tumor-Bearing Mice. Prostate cancer. 2014;2014:104248
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7-30
3. Mottet N, Bellmunt J, Bolla M, Briers E, Cumberbatch MG, De Santis M. et al. EAU-ESTRO-SIOG Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur Urol. 2017;71:618-29
4. Yossepowitch O, Briganti A, Eastham JA, Epstein J, Graefen M, Montironi R. et al. Positive surgical margins after radical prostatectomy: a systematic review and contemporary update. Eur Urol. 2014;65:303-13
5. Lutje S, Rijpkema M, Franssen GM, Fracasso G, Helfrich W, Eek A. et al. Dual-Modality Image-Guided Surgery of Prostate Cancer with a Radiolabeled Fluorescent Anti-PSMA Monoclonal Antibody. J Nucl Med. 2014;55:995-1001
6. Eiber M, Fendler WP, Rowe SP, Calais J, Hofman MS, Maurer T. et al. Prostate-Specific Membrane Antigen Ligands for Imaging and Therapy. J Nucl Med. 2017;58:67s-76s
7. Nagaya T, Nakamura YA, Choyke PL, Kobayashi H. Fluorescence-Guided Surgery. Front Oncol. 2017;7:314
8. Eastham JA, Kuroiwa K, Ohori M, Serio AM, Gorbonos A, Maru N. et al. Prognostic significance of location of positive margins in radical prostatectomy specimens. Urology. 2007;70:965-9
9. Heidenreich A, Bellmunt J, Bolla M, Joniau S, Mason M, Matveev V. et al. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and treatment of clinically localised disease. Eur Urol. 2011;59:61-71
10. Ananias HJ, van den Heuvel MC, Helfrich W, de Jong IJ. Expression of the gastrin-releasing peptide receptor, the prostate stem cell antigen and the prostate-specific membrane antigen in lymph node and bone metastases of prostate cancer. Prostate. 2009;69:1101-8
11. Lutje S, Heskamp S, Cornelissen AS, Poeppel TD, van den Broek SA, Rosenbaum-Krumme S. et al. PSMA Ligands for Radionuclide Imaging and Therapy of Prostate Cancer: Clinical Status. Theranostics. 2015;5:1388-401
12. Mannweiler S, Amersdorfer P, Trajanoski S, Terrett JA, King D, Mehes G. Heterogeneity of prostate-specific membrane antigen (PSMA) expression in prostate carcinoma with distant metastasis. Pathol Oncol Res. 2009;15:167-72
13. Perner S, Hofer MD, Kim R, Shah RB, Li H, Moller P. et al. Prostate-specific membrane antigen expression as a predictor of prostate cancer progression. Hum Pathol. 2007;38:696-701
14. Lutje S, Rijpkema M, Helfrich W, Oyen WJ, Boerman OC. Targeted radionuclide and fluorescence dual-modality imaging of cancer: preclinical advances and clinical translation. Mol Imaging Biol. 2014;16:747-55
15. Pinto JT, Suffoletto BP, Berzin TM, Qiao CH, Lin S, Tong WP. et al. Prostate-specific membrane antigen: a novel folate hydrolase in human prostatic carcinoma cells. Clin Cancer Res. 1996;2:1445-51
16. Pomper MG, Musachio JL, Zhang J, Scheffel U, Zhou Y, Hilton J. et al. 11C-MCG: synthesis, uptake selectivity, and primate PET of a probe for glutamate carboxypeptidase II (NAALADase). Mol Imaging. 2002;1:96-101
17. Tsukamoto T, Majer P, Vitharana D, Ni C, Hin B, Lu XC. et al. Enantiospecificity of glutamate carboxypeptidase II inhibition. J Med Chem. 2005;48:2319-24
18. Wu LY, Anderson MO, Toriyabe Y, Maung J, Campbell TY, Tajon C. et al. The molecular pruning of a phosphoramidate peptidomimetic inhibitor of prostate-specific membrane antigen. Bioorg Med Chem. 2007;15:7434-43
19. Kozikowski AP, Nan F, Conti P, Zhang J, Ramadan E, Bzdega T. et al. Design of remarkably simple, yet potent urea-based inhibitors of glutamate carboxypeptidase II (NAALADase). J Med Chem. 2001;44:298-301
20. Afshar-Oromieh A, Babich JW, Kratochwil C, Giesel FL, Eisenhut M, Kopka K. et al. The Rise of PSMA Ligands for Diagnosis and Therapy of Prostate Cancer. J Nucl Med. 2016;57:79s-89s
21. Robu S, Schottelius M, Eiber M, Maurer T, Gschwend J, Schwaiger M. et al. Preclinical Evaluation and First Patient Application of 99mTc-PSMA-I&S for SPECT Imaging and Radioguided Surgery in Prostate Cancer. J Nucl Med. 2017;58:235-42
22. Schottelius M, Wirtz M, Eiber M, Maurer T, Wester HJ. [(111)In]PSMA-I&T: expanding the spectrum of PSMA-I&T applications towards SPECT and radioguided surgery. EJNMMI Res. 2015;5:68
23. Maurer T, Robu S, Schottelius M, Schwamborn K, Rauscher I, van den Berg NS. et al. (99m)Technetium-based Prostate-specific Membrane Antigen-radioguided Surgery in Recurrent Prostate Cancer. Eur Urol. 2018
24. Horn T, Rauscher I, Eiber M, Gschwend JE, Maurer T. [PSMA-radioguided surgery in localised recurrent prostate cancer]. Urologe A. 2017;56:1417-23
25. Claeys T, Van Praet C, Lumen N, Ost P, Fonteyne V, De Meerleer G. et al. Salvage pelvic lymph node dissection in recurrent prostate cancer: surgical and early oncological outcome. Biomed Res Int. 2015;2015:198543
26. Knipper S, Tilki D, Mansholt J, Berliner C, Bernreuther C, Steuber T. et al. Metastases-yield and Prostate-specific Antigen Kinetics Following Salvage Lymph Node Dissection for Prostate Cancer: A Comparison Between Conventional Surgical Approach and Prostate-specific Membrane Antigen-radioguided Surgery. Eur Urol Focus. 2018
27. Porres D, Pfister D, Thissen A, Kuru TH, Zugor V, Buettner R. et al. The role of salvage extended lymph node dissection in patients with rising PSA and PET/CT scan detected nodal recurrence of prostate cancer. Prostate Cancer Prostatic Dis. 2017;20:85-92
28. Povoski SP, Neff RL, Mojzisik CM, O'Malley DM, Hinkle GH, Hall NC. et al. A comprehensive overview of radioguided surgery using gamma detection probe technology. World J Surg Oncol. 2009;7:11
29. Holl G, Dorn R, Wengenmair H, Weckermann D, Sciuk J. Validation of sentinel lymph node dissection in prostate cancer: experience in more than 2,000 patients. Eur J Nucl Med Mol Imaging. 2009;36:1377-82
30. Fortuin A, Rooij M, Zamecnik P, Haberkorn U, Barentsz J. Molecular and functional imaging for detection of lymph node metastases in prostate cancer. Int J Mol Sci. 2013;14:13842-75
31. KleinJan GH, van Werkhoven E, van den Berg NS, Karakullukcu MB, Zijlmans H, van der Hage JA. et al. The best of both worlds: a hybrid approach for optimal pre- and intraoperative identification of sentinel lymph nodes. Eur J Nucl Med Mol Imaging. 2018;45:1915-25
32. Brenot-Rossi I, Rossi D, Esterni B, Brunelle S, Chuto G, Bastide C. Radioguided sentinel lymph node dissection in patients with localised prostate carcinoma: influence of the dose of radiolabelled colloid to avoid failure of the procedure. Eur J Nucl Med Mol Imaging. 2008;35:32-8
33. Wengenmair H, Kopp J, Vogt H, Wawroschek F, Grober S, Dorn R. et al. [Sentinel lymph node diagnosis in prostatic carcinoma: II. Biokinetics and dosimetry of 99mTc-Nanocolloid after intraprostatic injection]. Nuklearmedizin. 2002;41:102-7
34. Bao K, Lee JH, Kang H, Park GK, El Fakhri G, Choi HS. PSMA-targeted contrast agents for intraoperative imaging of prostate cancer. Chem Commun (Camb). 2017;53:1611-4
35. Maurer T, Weirich G, Schottelius M, Weineisen M, Frisch B, Okur A. et al. Prostate-specific membrane antigen-radioguided surgery for metastatic lymph nodes in prostate cancer. Eur Urol. 2015;68:530-4
36. Rauscher I, Eiber M, Jilg CA, Gschwend JE, Maurer T. [PSMA-radioguided surgery in localized recurrent prostate cancer: Current and future aspects]. Urologe A. 2017;56:18-23
37. Rauscher I, Horn T, Eiber M, Gschwend JE, Maurer T. Novel technology of molecular radio-guidance for lymph node dissection in recurrent prostate cancer by PSMA-ligands. World J Urol. 2018;36:603-8
38. Bugby SL, Lees JE, Perkins AC. Hybrid intraoperative imaging techniques in radioguided surgery: present clinical applications and future outlook. Clin Transl Imaging. 2017;5:323-41
39. Jones AD, Wilton JC. Can intra-operative fluorescence play a significant role in hepatobiliary surgery? Eur J Surg Oncol. 2017;43:1622-7
40. Vahrmeijer AL, Hutteman M, van der Vorst JR, van de Velde CJ, Frangioni JV. Image-guided cancer surgery using near-infrared fluorescence. Nat Rev Clin Oncol. 2013;10:507-18
41. Fomina N, McFearin CL, Sermsakdi M, Morachis JM, Almutairi A. Low power, biologically benign NIR light triggers polymer disassembly. Macromolecules. 2011;44:8590-7
42. Bai M, Bornhop DJ. Recent advances in receptor-targeted fluorescent probes for in vivo cancer imaging. Curr Med Chem. 2012;19:4742-58
43. Humblet V, Lapidus R, Williams LR, Tsukamoto T, Rojas C, Majer P. et al. High-affinity near-infrared fluorescent small-molecule contrast agents for in vivo imaging of prostate-specific membrane antigen. Mol Imaging. 2005;4:448-62
44. Wang X, Huang SS, Heston WD, Guo H, Wang BC, Basilion JP. Development of targeted near-infrared imaging agents for prostate cancer. Mol Cancer Ther. 2014;13:2595-606
45. Huang SS, Wang X, Zhang Y, Doke A, DiFilippo FP, Heston WD. Improving the biodistribution of PSMA-targeting tracers with a highly negatively charged linker. Prostate. 2014;74:702-13
46. Banerjee SR, Foss CA, Castanares M, Mease RC, Byun Y, Fox JJ. et al. Synthesis and evaluation of technetium-99m- and rhenium-labeled inhibitors of the prostate-specific membrane antigen (PSMA). J Med Chem. 2008;51:4504-17
47. Chen Y, Pullambhatla M, Banerjee SR, Byun Y, Stathis M, Rojas C. et al. Synthesis and biological evaluation of low molecular weight fluorescent imaging agents for the prostate-specific membrane antigen. Bioconjug Chem. 2012;23:2377-85
48. Benesova M, Schafer M, Bauder-Wust U, Afshar-Oromieh A, Kratochwil C, Mier W. et al. Preclinical Evaluation of a Tailor-Made DOTA-Conjugated PSMA Inhibitor with Optimized Linker Moiety for Imaging and Endoradiotherapy of Prostate Cancer. J Nucl Med. 2015;56:914-20
49. Eder M, Schafer M, Bauder-Wust U, Hull WE, Wangler C, Mier W. et al. 68Ga-complex lipophilicity and the targeting property of a urea-based PSMA inhibitor for PET imaging. Bioconjug Chem. 2012;23:688-97
50. Benesova M, Umbricht CA, Schibli R, Muller C. Albumin-Binding PSMA Ligands: Optimization of the Tissue Distribution Profile. Mol Pharm. 2018;15:934-46
51. Kularatne SA, Thomas M, Myers CH, Gagare P, Kanduluru AK, Crian CJ. et al. Evaluation of Novel Prostate-Specific Membrane Antigen-Targeted Near-Infrared Imaging Agent for Fluorescence-Guided Surgery of Prostate Cancer. Clin Cancer Res. 2018
52. Kelderhouse LE, Chelvam V, Wayua C, Mahalingam S, Poh S, Kularatne SA. et al. Development of tumor-targeted near infrared probes for fluorescence guided surgery. Bioconjug Chem. 2013;24:1075-80
53. Neuman BP, Eifler JB, Castanares M, Chowdhury WH, Chen Y, Mease RC. et al. Real-time, near-infrared fluorescence imaging with an optimized dye/light source/camera combination for surgical guidance of prostate cancer. Clin Cancer Res. 2015;21:771-80
54. Baranski AC, Schafer M, Bauder-Wust U, Roscher M, Schmidt J, Stenau E. et al. PSMA-11 Derived Dual-labeled PSMA-Inhibitors for Preoperative PET Imaging and Precise Fluorescence-Guided Surgery of Prostate Cancer. J Nucl Med. 2017
55. Banerjee SR, Foss CA, Horhota A, Pullambhatla M, McDonnell K, Zale S. et al. (111)In- and IRDye800CW-Labeled PLA-PEG Nanoparticle for Imaging Prostate-Specific Membrane Antigen-Expressing Tissues. Biomacromolecules. 2017;18:201-9
56. Kommidi H, Guo H, Nurili F, Vedvyas Y, Jin MM, McClure TD. et al. (18)F-Positron Emitting/Trimethine Cyanine-Fluorescent Contrast for Image-Guided Prostate Cancer Management. J Med Chem. 2018
57. Wustemann T, Haberkorn U, Babich J, Mier W. Targeting prostate cancer: Prostate-specific membrane antigen based diagnosis and therapy. Med Res Rev. 2018
58. Banerjee SR, Pullambhatla M, Byun Y, Nimmagadda S, Foss CA, Green G. et al. Sequential SPECT and optical imaging of experimental models of prostate cancer with a dual modality inhibitor of the prostate-specific membrane antigen. Angew Chem Int Ed Engl. 2011;50:9167-70
59. Schottelius M, Wurzer A, Wissmiller K, Beck R, Koch M, Gkorpas D. et al. Synthesis and preclinical characterization of the PSMA-targeted hybrid tracer PSMA-I&F for nuclear and fluorescence imaging of prostate cancer. J Nucl Med. 2018
60. Hekman MC, Rijpkema M, Muselaers CH, Oosterwijk E, Hulsbergen-Van de Kaa CA, Boerman OC. et al. Tumor-targeted Dual-modality Imaging to Improve Intraoperative Visualization of Clear Cell Renal Cell Carcinoma: A First in Man Study. Theranostics. 2018;8:2161-70
61. Liu T, Wu LY, Choi JK, Berkman CE. In vitro targeted photodynamic therapy with a pyropheophorbide-a conjugated inhibitor of prostate-specific membrane antigen. Prostate. 2009;69:585-94
62. Liu T, Wu LY, Berkman CE. Prostate-specific membrane antigen-targeted photodynamic therapy induces rapid cytoskeletal disruption. Cancer Lett. 2010;296:106-12
63. Liu T, Wu LY, Choi JK, Berkman CE. Targeted photodynamic therapy for prostate cancer: inducing apoptosis via activation of the caspase-8/-3 cascade pathway. Int J Oncol. 2010;36:777-84
64. Harmatys KM, Overchuk M, Chen J, Ding L, Chen Y, Pomper MG. et al. Tuning Pharmacokinetics to Improve Tumor Accumulation of a Prostate-Specific Membrane Antigen-Targeted Phototheranostic Agent. Bioconjug Chem. 2018;29:3746-56
65. Chen Y, Chatterjee S, Lisok A, Minn I, Pullambhatla M, Wharram B. et al. A PSMA-targeted theranostic agent for photodynamic therapy. J Photochem Photobiol B. 2017;167:111-6
66. Wang X, Tsui B, Ramamurthy G, Zhang P, Meyers J, Kenney ME. et al. Theranostic Agents for Photodynamic Therapy of Prostate Cancer by Targeting Prostate-Specific Membrane Antigen. Mol Cancer Ther. 2016;15:1834-44
67. Watanabe R, Hanaoka H, Sato K, Nagaya T, Harada T, Mitsunaga M. et al. Photoimmunotherapy targeting prostate-specific membrane antigen: are antibody fragments as effective as antibodies? J Nucl Med. 2015;56:140-4
68. Lütje S, Heskamp S, Franssen GM, Frielink C, Kip A, Hekman M. et al. Development and characterization of a theranostic multimodal anti-PSMA targeting agent for imaging, surgical guidance, and targeted photodynamic therapy of PSMA-expressing tumors. Theranostics. 2019;9(10):2924-38
69. Horoszewicz JS, Kawinski E, Murphy GP. Monoclonal antibodies to a new antigenic marker in epithelial prostatic cells and serum of prostatic cancer patients. Anticancer Res. 1987;7:927-35
70. Liu H, Rajasekaran AK, Moy P, Xia Y, Kim S, Navarro V. et al. Constitutive and antibody-induced internalization of prostate-specific membrane antigen. Cancer Res. 1998;58:4055-60
71. Hillier SM, Maresca KP, Femia FJ, Marquis JC, Foss CA, Nguyen N. et al. Preclinical evaluation of novel glutamate-urea-lysine analogues that target prostate-specific membrane antigen as molecular imaging pharmaceuticals for prostate cancer. Cancer Res. 2009;69:6932-40
72. Kopka K, Benesova M, Barinka C, Haberkorn U, Babich J. Glu-Ureido-Based Inhibitors of Prostate-Specific Membrane Antigen: Lessons Learned During the Development of a Novel Class of Low-Molecular-Weight Theranostic Radiotracers. J Nucl Med. 2017;58:17s-26s
73. Lindenberg L, Choyke P, Dahut W. Prostate Cancer Imaging with Novel PET Tracers. Curr Urol Rep. 2016;17:18
74. Zwicke GL, Mansoori GA, Jeffery CJ. Utilizing the folate receptor for active targeting of cancer nanotherapeutics. Nano Rev. 2012:3
75. Garai I, Barna S, Nagy G, Forgacs A. Limitations and pitfalls of 99mTc-EDDA/HYNIC-TOC (Tektrotyd) scintigraphy. Nucl Med Rev Cent East Eur. 2016;19:93-8
76. Love C, Palestro CJ. Radionuclide imaging of infection. J Nucl Med Technol. 2004;32:47-57 quiz 8-9
77. Alam IS, Steinberg I, Vermesh O, van den Berg NS, Rosenthal EL, van Dam GM. et al. Emerging Intraoperative Imaging Modalities to Improve Surgical Precision. Mol Imaging Biol. 2018;20:705-15
78. Orsaria P, Chiaravalloti A, Fiorentini A, Pistolese C, Vanni G, Granai AV. et al. PET Probe-Guided Surgery in Patients with Breast Cancer: Proposal for a Methodological Approach. In vivo. 2017;31:101-10
79. Polom K, Murawa D, Rho YS, Nowaczyk P, Hunerbein M, Murawa P. Current trends and emerging future of indocyanine green usage in surgery and oncology: a literature review. Cancer. 2011;117:4812-22
80. Tynan CJ, Clarke DT, Coles BC, Rolfe DJ, Martin-Fernandez ML, Webb SE. Multicolour single molecule imaging in cells with near infra-red dyes. PloS one. 2012;7:e36265
81. Lutje S, Slavik R, Fendler W, Herrmann K, Eiber M. PSMA ligands in prostate cancer - Probe optimization and theranostic applications. Methods. 2017;130:42-50
82. van Leeuwen FWB, van Oosterom MN, Meershoek P, van Leeuwen PJ, Berliner C, van der Poel HG. et al. Minimal-Invasive Robot-Assisted Image-Guided Resection of Prostate-Specific Membrane Antigen-Positive Lymph Nodes in Recurrent Prostate Cancer. Clin Nucl Med. 2019;44:580-1
83. van Oosterom MN, Simon H, Mengus L, Welling MM, van der Poel HG, van den Berg NS. et al. Revolutionizing (robot-assisted) laparoscopic gamma tracing using a drop-in gamma probe technology. Am J Nucl Med Mol Imaging. 2016;6:1-17
84. Meershoek P, van Oosterom MN, Simon H, Mengus L, Maurer T, van Leeuwen PJ. et al. Robot-assisted laparoscopic surgery using DROP-IN radioguidance: first-in-human translation. Eur J Nucl Med Mol Imaging. 2019;46:49-53
85. Chang SS, O'Keefe DS, Bacich DJ, Reuter VE, Heston WD, Gaudin PB. Prostate-specific membrane antigen is produced in tumor-associated neovasculature. Clin Cancer Res. 1999;5:2674-81
86. Chang SS, Reuter VE, Heston WD, Bander NH, Grauer LS, Gaudin PB. Five different anti-prostate-specific membrane antigen (PSMA) antibodies confirm PSMA expression in tumor-associated neovasculature. Cancer Res. 1999;59:3192-8
87. Chang SS, Reuter VE, Heston WD, Gaudin PB. Metastatic renal cell carcinoma neovasculature expresses prostate-specific membrane antigen. Urology. 2001;57:801-5
88. Haffner MC, Kronberger IE, Ross JS, Sheehan CE, Zitt M, Muhlmann G. et al. Prostate-specific membrane antigen expression in the neovasculature of gastric and colorectal cancers. Hum Pathol. 2009;40:1754-61
89. Sasikumar A, Kashyap R, Joy A, Charan Patro K, Bhattacharya P, Reddy Pilaka VK. et al. Utility of 68Ga-PSMA-11 PET/CT in Imaging of Glioma-A Pilot Study. Clin Nucl Med. 2018;43:e304-e9
90. Meyer AR, Carducci MA, Denmeade SR, Markowski MC, Pomper MG, Pierorazio PM. et al. Improved identification of patients with oligometastatic clear cell renal cell carcinoma with PSMA-targeted (18)F-DCFPyL PET/CT. Ann Nucl Med. 2019
Author contact
![]() Corresponding author: Yvonne Derks, Department of Radiology and Nuclear Medicine, Radboud University Medical Center, Geert Grooteplein Zuid 10, 6525GA Nijmegen, Phone: +31 (0)24 365 5340, E-mail: yvonne.derksnl
Corresponding author: Yvonne Derks, Department of Radiology and Nuclear Medicine, Radboud University Medical Center, Geert Grooteplein Zuid 10, 6525GA Nijmegen, Phone: +31 (0)24 365 5340, E-mail: yvonne.derksnl
 Global reach, higher impact
Global reach, higher impact