13.3
Impact Factor
Theranostics 2019; 9(23):6809-6823. doi:10.7150/thno.36988 This issue Cite
Research Paper
Self-generating oxygen enhanced mitochondrion-targeted photodynamic therapy for tumor treatment with hypoxia scavenging
1. Department of General Surgery, Nanjing Drum Tower Hospital, The Affiliated Hospital of Nanjing University Medical School, No. 321 Zhongshan Road, Nanjing, 210008, China.
2. School of Biological Science and Medical Engineering, Southeast University, No. 87 Dingjiaqiao, Nanjing, 210009, China
3. Department of Radiology, Nanjing Drum Tower Hospital, The Affiliated Hospital of Nanjing University Medical School, No. 321 Zhongshan Road, Nanjing, 210008, China.
*These authors contributed equally to this work.
Abstract
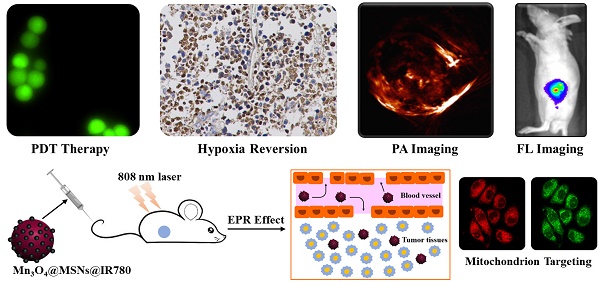
Tumor hypoxia is an important reason for the limited therapeutic efficacy of photodynamic therapy (PDT) because of the oxygen requirement of the therapeutic process. PDT consumes tissue oxygen and destroys tumor vasculature, further hampering its own efficacy in promoting tumor deterioration. Therefore, overcoming the photodynamic exacerbation of tumor hypoxia is urgent.
Methods: Herein, we report a photodynamic nanoparticle with sustainable hypoxia remission skills by both intratumoral H2O2 catalysis and targeted mitochondrial destruction. The Mn3O4@MSNs@IR780 nanoparticles are formed by absorbing a photosensitizer (IR780) into 90 nm mesoporous silica nanoparticles (MSNs) and capping the surface pores with 5 nm Mn3O4 nanoparticles.
Results: These Mn3O4 nanoparticles can accumulate in tumors and respond to the H2O2-enriched tumor microenvironment by decomposing and catalyzing H2O2 into O2. Afterwards, IR780 is released and activated, spontaneously targeting the mitochondria due to its natural mitochondrial affinity. Under laser irradiation, this self-generated oxygen-enhanced PDT can destroy mitochondria and inhibit cell respiration, resulting in sustainable hypoxia remission in tumor tissues and consequently enhancing the therapeutic outcome. In vitro experiments suggest that Mn3O4@MSNs@IR780 exhibited highly mitochondrion-targeted properties and could sustainably inhibit tumor hypoxia. Additionally, the highest photoacoustic signal of HbO2 with the lowest Hb was observed in tumors from mice after PDT, indicating that these nanoparticles can also prevent tumor hypoxia in vivo.
Conclusion: Taken together, our study indicated a new approach for overcoming the sustainable hypoxia limitation in traditional PDT by targeted oxygen supplementation and mitochondria destruction.
Keywords: mitochondrion targeting, endogenous oxygen generation, photodynamic therapy, tumor hypoxia, NIR fluorescence imaging.
 Global reach, higher impact
Global reach, higher impact