13.3
Impact Factor
Theranostics 2019; 9(21):6047-6062. doi:10.7150/thno.36378 This issue Cite
Research Paper
Exosome-dependent immune surveillance at the metastatic niche requires BAG6 and CBP/p300-dependent acetylation of p53
1. Experimental Tumor Research, Center for Tumor Biology and Immunology, Clinic for Hematology, Oncology and Immunology, Philipps University, Marburg, Germany
2. Innate Immunity Group, Department I of Internal Medicine, University Hospital of Cologne, Cologne, Germany
3. Institute of Clinical Chemistry and Clinical Pharmacology, University Hospital Bonn, Bonn, Germany
4. Institute of Molecular Biology and Tumor Research (IMT), Center for Tumor Biology and Immunology (ZTI), Philipps University, Marburg, Germany
5. Center for Tumor Biology and Immunology (ZTI), Core Facility Cellular Imaging, Philipps University, Marburg, Germany
6. Cancer and Immunometabolism Research Group, Department I of Internal Medicine, University Hospital of Cologne, Germany (current address: Department of Medicine III, University Hospital Munich, LMU, Germany)
7. Institute of Molecular Oncology and Genomics Core Facility, Center for Tumor Biology and Immunology (ZTI), Philipps University Marburg, Germany
8. Department of Dermatology, University Hospital Essen, Essen and German Cancer Consortium (DKTK), Partner site University Hospital Essen, Essen, Germany
9. Clinic I of Internal Medicine, University Hospital of Cologne, Cologne, Germany.
10. Cologne Excellence Cluster on Cellular Stress Response in Aging-Associated Diseases (CECAD), University of Cologne, Cologne, Germany.
11. Center of Integrated Oncology (CIO), University Hospital of Cologne, Cologne, Germany.
12. Center for Molecular Medicine Cologne (CMMC), University of Cologne, Cologne, Germany
Abstract
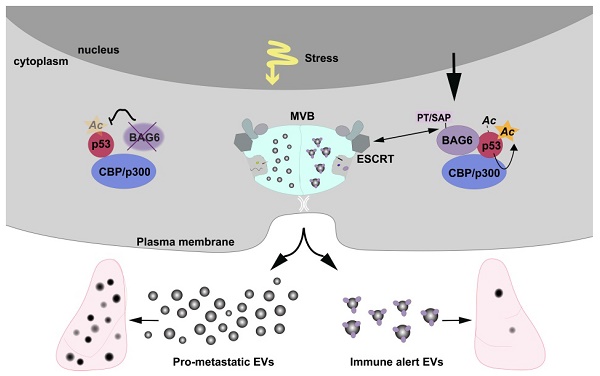
Extracellular vesicles released by tumor cells contribute to the reprogramming of the tumor microenvironment and interfere with hallmarks of cancer including metastasis. Notably, melanoma cell-derived EVs are able to establish a pre-metastatic niche in distant organs, or on the contrary, exert anti-tumor activity. However, molecular insights into how vesicles are selectively packaged with cargo defining their specific functions remain elusive.
Methods: Here, we investigated the role of the chaperone Bcl2-associated anthogene 6 (BAG6, synonym Bat3) for the formation of pro- and anti-tumor EVs. EVs collected from wildtype cells and BAG6-deficient cells were characterized by mass spectrometry and RNAseq. Their tumorigenic potential was analyzed using the B-16V transplantation mouse melanoma model.
Results: We demonstrate that EVs from B-16V cells inhibit lung metastasis associated with the mobilization of Ly6Clow patrolling monocytes. The formation of these anti-tumor-EVs was dependent on acetylation of p53 by the BAG6/CBP/p300-acetylase complex, followed by recruitment of components of the endosomal sorting complexes required for transport (ESCRT) via a P(S/T)AP double motif of BAG6. Genetic ablation of BAG6 and disruption of this pathway led to the release of a distinct EV subtype, which failed to suppress metastasis but recruited tumor-promoting neutrophils to the pre-metastatic niche.
Conclusion: We conclude that the BAG6/CBP/p300-p53 axis is a key pathway directing EV cargo loading and thus a potential novel microenvironmental therapeutic target.
Keywords: extracellular vesicles, exosomes, BAG6/CBP/p300-p53, metastasis, melanoma
 Global reach, higher impact
Global reach, higher impact