13.3
Impact Factor
Theranostics 2019; 9(19):5681-5693. doi:10.7150/thno.34824 This issue Cite
Research Paper
Heterologous and cross-species tropism of cancer-derived extracellular vesicles
1. Department of Oncology and Hemato-Oncology, Center of Excellence on Neurodegenerative Diseases, University of Milan, Milan, Italy
2. Department of Pharmacological and Biomolecular Sciences, University of Milan, Milan, Italy
3. Targovax Oy, Clinical Science, Helsinki, Finland
4. National Institute of Public Health - National Institute of Hygiene, Department of Virology, Warsaw, Poland
5. Istituto Nazionale Tumori Fondazione IRCCS, Milan, Italy
Abstract
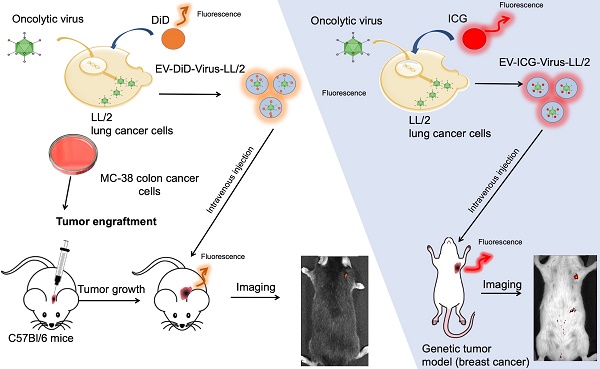
Extracellular vesicles (EVs) are naturally occurring cargo delivery vesicles that have recently received considerable attention for their roles in intercellular communication in many physiological and pathological processes, including tumourigenesis. EVs generated by different tissues demonstrated specific homing: in particular, cancer-derived EVs showed a selective tropism for the tumor tissue from which the vesicles originated. For this property, EVs have been proposed as drug delivery tools for anti-cancer therapies, although the limited knowledge about their in vivo tropism hinders their therapeutic applications. The current study aimed to characterize the targeting properties of cancer-derived EVs in vitro and their biodistribution in vivo, by using an imaging approach.
Methods: EVs were generated from: i) murine lung (LL/2) and colon (MC-38) cancer lines, ii) human lung cancer cell line (A549) and iii) human liver biopsy samples from healthy individuals. EVs were loaded with fluorescent dyes alone or in combination with a biopharmaceutical agent, the oncolytic adenovirus (OV), characterized for charge and size and tested for their activity in cancer cell lines. Finally, optical imaging was extensively applied to study in vivo and ex vivo the biodistribution of EVs originated from different sources in different mouse models of cancer, including xenograft, syngeneic graft and the MMTV-NeuT genetically modified animal.
Results: We initially demonstrated that even loading EVs even with a large biopharmaceutical oncolytic viruses (OVs) did not significantly change their charge and dimension properties, while increasing their anti-neoplastic activity compared to the virus or EVs alone. Interestingly, this activity was observed even if the EVs derived from lung cancer were applied to colon carcinoma cell lines and vice versa, suggesting that the EV uptake occurred in vitro without any specificity for the cancer cells from which the vesicles originated. When administered i.v (intravenously) to the mouse models of cancer, the tumour-derived EVs, but not the EVs derived from a healthy tissue, demonstrated a selective accumulation of the fluorescence at the tumour site 24 h after injection; adding OVs to the formulation did not change the tumour-specific tropism of the EVs also in vivo. Most interestingly, the in vivo experiments confirmed the in vitro observation of the generalized tropism of tumour-derived EVs for any neoplastic tissue, independent of the tumour type or even the species originating the vesicles.
Conclusions: Taken together, our in vitro and in vivo data demonstrate for the first time a heterologous, cross-species tumour-tropism for cancer-derived EVs. This finding challenges our current view on the homing properties of EVs and opens new avenues for the selective delivery of diagnostic/therapeutic agents to solid tumours.
Keywords: extracellular vesicles, oncolytic adenoviruses, cancer therapy, immunocompetent cancer mouse models, in vivo imaging.
 Global reach, higher impact
Global reach, higher impact