13.3
Impact Factor
Theranostics 2019; 9(19):5626-5641. doi:10.7150/thno.34778 This issue Cite
Research Paper
Evaluation of novel formulations for transarterial chemoembolization: combining elements of Lipiodol emulsions with Drug-eluting Beads
1. Biocompatibles UK Ltd (a BTG International group company), Lakeview, Riverside Way, Watchmoor Park, Camberley, Surrey, GU15 3YL, UK.
2. Gustave Roussy 114, rue Edouard Vaillant, 94805, Villejuif, France
Abstract
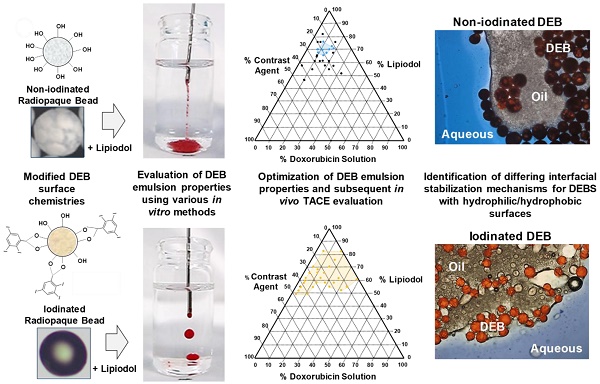
There are currently two methods widely used in clinical practice to perform transarterial chemoembolization (TACE). One is based on mixing an aqueous drug with an iodized oil (Lipiodol) and creating an emulsion that is delivered intraarterially, followed by embolization with a particulate agent. The other is based on a one-step TACE using Drug-eluting Beads (DEBs) loaded with drug. It is not recommended to mix Lipiodol with DEBs due to incompatibility. For the first time, novel DEB: Lipiodol: doxorubicin (Dox) emulsions are identified using lyophilized polyvinyl alcohol (PVA) hydrogels (non-iodinated or iodinated) DEBs.
Methods: 15 DEB emulsions (50mg Dox) were assessed for stability and deliverability in vitro and in vivo in a swine model. Dox release from selected formulations was measured in vitro using a vascular flow model and in vivo in a VX2 rabbit tumor model.
Results: Both DEB formats were shown to be able to form emulsions, however only Iodinated DEBs consistently met defined handling criteria. Those based on the non-iodinated DEB achieved >99%+ Dox loading in <5 minutes but were generally less stable. Those prepared using iodinated DEBs, which are more hydrophobic, were able to form stable Pickering-like emulsions (separation time ≥ 20 minutes) and demonstrated handling, administration and imaging observations more akin to Lipiodol™ TACE emulsions in both embolization models. Controlled Dox release and hence beneficial in vivo pharmacokinetics associated with DEB-TACE were maintained.
Conclusions: This study demonstrates that it is possible to formulate novel DEB emulsions suitable for TACE that combine positive elements of both Lipiodol™ based and DEB-TACE procedures.
Keywords: Transarterial chemoembolization, Drug-loaded emulsions, in vitro-in vivo correlation.
 Global reach, higher impact
Global reach, higher impact