13.3
Impact Factor
Theranostics 2019; 9(14):4019-4029. doi:10.7150/thno.33000 This issue Cite
Review
New insights into the pathogenesis and treatment of sarcopenia in chronic heart failure
1. Department of Cardiology, The Second Affiliated Hospital of Soochow University, Suzhou, China
2. Department of Geriatrics, Sir Run Run Hospital Affiliated to Nanjing Medical University, Nanjing, China
3. Department of Neurosurgery, The Second Affiliated Hospital of Soochow University, Suzhou, China
# These authors contributed equally to this work.
Received 2019-1-10; Accepted 2019-4-10; Published 2019-5-31
Abstract
Sarcopenia is an age-related geriatric syndrome that is characterized by a progressive loss of muscle mass, strength and function. Chronic heart failure (CHF), the final stage of various cardiovascular diseases, may be closely correlated with the occurrence of sarcopenia. Accumulating evidence has demonstrated that CHF can promote the development of sarcopenia through multiple pathophysiological mechanisms, including malnutrition, inflammation, hormonal changes, oxidative stress, autophagy, and apoptosis. Additionally, CHF can aggravate the adverse outcomes associated with sarcopenia, including falls, osteoporosis, frailty, cachexia, hospitalization, and mortality. Sarcopenia and CHF are mutually interacting clinical syndromes. Patients with these two syndromes seem to endure a double burden, with no particularly effective way to hinder their progression. However, the combination of physical exercise, nutritional supplements, and drug therapy may counteract the development of these maladies. In this review, we will summarize the latest progress in the pathogenesis and treatment of sarcopenia in patients with CHF.
Keywords: chronic heart failure, pathogenesis, sarcopenia, treatment
1. Introduction
With an increasing proportion of the population being of advanced age, improving quality of life for the elderly has become a major challenge for geriatricians. The elderly often suffer from various geriatric syndromes, among which sarcopenia has been recognized as a new disease and has attracted significant worldwide attention [1]. In recent years, the definition and diagnosis of sarcopenia have been continuously updated, and there is no consistent international standard. The current definition of sarcopenia is age-related loss of skeletal muscle quantity or quality and a decline in muscle strength and/or physical performance [2]. The prevalence of sarcopenia ranges from 5 to 13% in persons aged 60 to 70 years and may even reach 50% in octogenarians, most of whom suffer from adverse events including falls, osteoporosis, a decline in quality of life, and increased mortality [3].
Chronic heart failure (CHF), another important geriatric syndrome, which is caused by cardiac contractile or diastolic dysfunction due to various cardiovascular disorders, including ischemic heart disease, hypertension, and cardiomyopathies [4]. Cardiac pump dysfunction can reduce cardiac output, increase venous pressure, and is accompanied by ventricular remodeling, which causes progressive deterioration of the failing heart. Sarcopenia is a frequent co-morbidity among patients with CHF, which may adversely affect the prognosis of patients [5]. The results of SICA-HF study showed that the prevalence of sarcopenia in CHF patients was nearly 20% higher than healthy individuals [6]. Loss of peripheral skeletal muscle occurs early in most CHF patients regardless of reduced or preserved ejection fraction, which is closely related to a decline in physical activity [7].
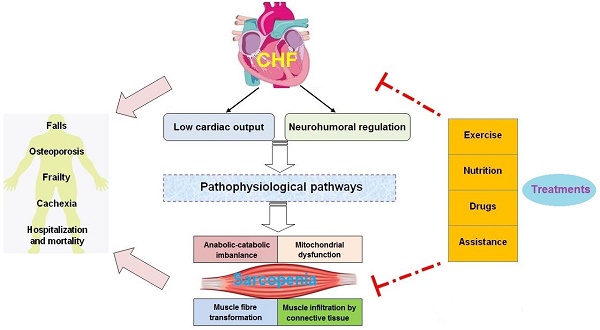
Recently, accumulating evidence has suggested that CHF and sarcopenia can interact with each other and contribute to reduced physiological function and increased mortality in elderly patients. Although significant progress has been made in the pathological mechanisms of these two syndromes, there is still a lack of effective treatment for CHF patients with sarcopenia. This review will focus on the new insights into the pathogenesis and treatment of sarcopenia in CHF. We clarify how pathophysiological changes associated with CHF affect the muscle mass, structure, and function. In addition, we discuss how CHF aggravates the adverse outcomes of sarcopenia, including falls, osteoporosis, frailty, cachexia, hospitalization and mortality, which can facilitate the comprehensive management for patients with CHF and sarcopenia (Figure 1). Furthermore, we summarize the current treatment perspectives of these two geriatric syndromes. We not only emphasize the importance of physical exercise, nutritional supplements, and drug therapies, but also discuss the application prospects of some assistive technologies (Figure 2).
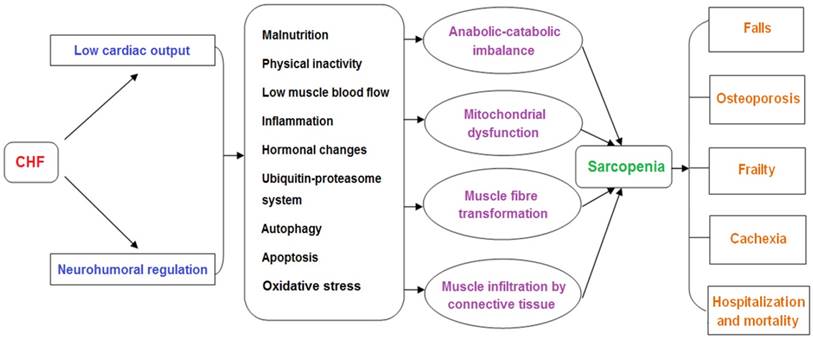
Pathogenesis of sarcopenia in chronic heart failure
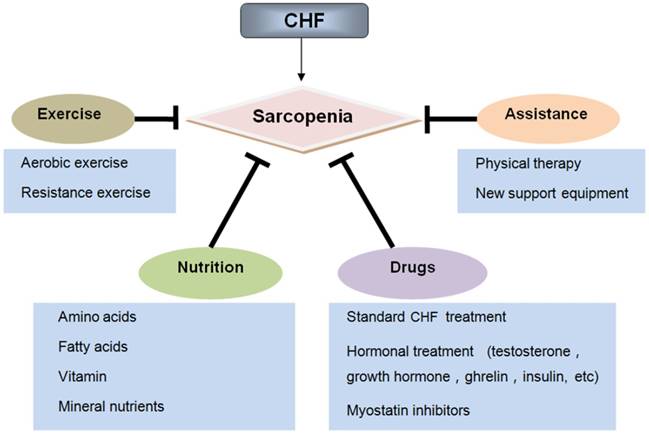
Treatment of sarcopenia in chronic heart failure
2. Pathogenesis of sarcopenia in CHF
In CHF, decreased cardiac output and systemic congestion lead to reduced food intake and exercise capacity, promote the release of inflammatory factors, increase sympathetic excitability, and affect muscle-related hormone secretion. These factors work together in muscle tissue, resulting in a decline of skeletal muscle growth factor and increased oxidative damage, which enhances activity of the ubiquitin-proteasome system (UPS) and induces autophagy and apoptosis. These changes contribute to an imbalance in muscle protein synthesis and degradation, causing skeletal muscle wasting.
Skeletal muscle dysfunction in sarcopenia and CHF has some common molecular signatures but others are distinguished, and the direct causality remains unelucidated. For instance, the fiber type switch from type II to type I is observed in sarcopenia but type I atrophy is shown to occur in CHF. Although muscle mitochondrial dysfunction with regard to quality and quantity is a distinct feature in CHF, the direct evidence between such mitochondrial abnormalities and skeletal muscle atrophy remains undetermined.
2.1. Malnutrition
The etiology of sarcopenia in patients with CHF is multifactorial, and malnutrition is involved in its pathogenesis. In CHF, an elevated resting energy expenditure has been shown, and the negative balance between energy demand and expenditure leads to a catabolic state and causes protein-energy malnutrition [8, 9]. Anorexia, a common symptom in patients with CHF, is independently associated with decreased muscle mass and strength [10]. The causes of anorexia are complex and the physiological changes are diverse. Patients with CHF often have pulmonary and gastrointestinal edema, which can contribute to dysgeusia, nausea, and gastroenteropathy, eventually causing anorexia and malabsorption [11]. In addition, some therapeutic drugs for CHF, especially digoxin, are also potential causes of anorexia [12].
2.2. Physical inactivity
Age-related decline in exercise capacity is the main factor for the loss of muscle mass and strength [13]. Additionally, physical inactivity and long bed rest often exist in CHF patients and are associated with muscle wasting and dysfunction. Inactivity can attenuate the muscle protein synthesis through impairing mTORC1 signaling and amino acid transporter expression [14]. Moreover, insulin sensitivity is also decreased in the elderly with long bed rest, which further negatively affects muscle homeostasis [15]. Inflammation may also be associated with physical inactivity. The levels of inflammatory factors are significantly elevated in elderly patients with long bed rest, suggesting that higher inflammatory status may be an important factor for muscle catabolism following physical inactivity [16].
2.3. Low muscle blood flow
In CHF, low muscle blood flow, as evaluated by capillary density, is a potentially significant factor for lower muscle performance. Decreased cardiac output results in a decline in skeletal muscle blood flow and consequently affect muscle mass and strength. For patients with CHF, exercise can aggravate tissue ischemia and promote lactate accumulation. Low baseline and peak reactive hyperemia blood flow in forearm and leg often occurs in CHF, suggesting that CHF patients are more likely to suffer from endothelial dysfunction, which is one of the mechanisms of sarcopenia [17]. Ischemic exercise can increase collateral blood flow in skeletal muscle. Blood flow restricted exercise training is effective in increasing muscular strength and size in older adults [18].
2.4. Inflammation
Patients with CHF often have chronic low-level systemic inflammation, which may exert sustained effects on skeletal muscle. Inflammatory mediators released into circulation further activate systemic inflammation and promote muscle atrophy. Elevated levels of inflammatory markers such as tumor necrosis factor-alpha, C-reactive protein, and interleukin-6 are correlated with decline in muscle mass and strength, which suggests a weight-associated pathway for inflammation in sarcopenia [19]. Moreover, Sarcopenic obesity can promote the release of pro-inflammatory cytokines, which in turn negatively affect muscle mass and strength [20]. Thus, low-grade inflammation contributes to skeletal muscle atrophy and dysfunction in patients with CHF.
2.5. Hormonal changes
In CHF, the decline in anabolic hormones and increase in catabolic hormones seem to be associated with sarcopenia. Insulin-like growth factor 1 (IGF-1) is a ligand necessary for growth hormone to produce physiological effects. Decrease in growth hormone and IGF-1 levels can lead to poor physical performance and sarcopenia [21, 22]. Aerobic exercise training-induced activation of IGF-I/Akt/mTOR signaling pathway could counteract muscle wasting in heart failure mice [23]. Angiotensin is elevated by neurohumoral compensation in patients with CHF and plays an important role in the pathological process of sarcopenia via multiple mechanisms. Angiotensin II infusion can induce muscle wasting via altered IGF-1 signaling, increased apoptosis, enhanced muscle protein breakdown, and reduced appetite [24]. Patients with CHF often have low testosterone levels [25], which is associated with muscle mass loss, functional impairment, and adverse outcomes [26-28]. In the cytoplasm, testosterone binds to androgen receptors and promotes protein transcription via the mitogen-activated protein kinase pathway, which increases muscle protein synthesis and muscle mass [29]. Myostatin is a member of the transforming growth factor beta family and negatively regulates muscle mass. Myostatin inhibition enhances the effects of exercise on performance and metabolic outcomes in aged mice [30]. Myostatin has also been shown to be significantly upregulated in CHF patients [31]. Elderly patients with CHF usually have lower ghrelin levels, which is a peptide produced in the stomach with various activities, including regulating appetite and promoting food intake and growth hormone release [32]. Thus, the decreased level of ghrelin is associated with the occurrence of sarcopenia.
2.6. The UPS
In CHF, one of the main features of skeletal muscle atrophy is an imbalance of myofibrillar protein levels, probably due to increased protein degradation [33]. UPS is the primary mechanism of protein degradation, and includes a coordinated enzymatic system of activation, binding, and protein targeting with ubiquitin, which is further degraded by proteasomes [34]. This process is performed by a group of enzymes called E3 ubiquitin ligases, which recognize protein substrates to be ubiquitinated by the action of E2 enzymes. Atrogin-1 and MuRF-1 are two E3 ubiquitin ligases that are important regulators of ubiquitinmediated protein degradation in skeletal muscle. They are induced in response to myostatin/ transforming growth factor-β signaling and are critically involved in the pathogenesis of sarcopenia [35].
2.7. Autophagy
Autophagy activation is essential for cellular homeostasis by preventing accumulation of metabolic waste products. In CHF, the inability to remove damaged structures leads to increased oxidative stress, reduced ATP production, collapse of the cellular catabolic machinery, and cell death [36]. Autophagy is a degradation pathway crucial for the removal of dysfunctional organelles and damaged macromolecules during aging process. Overweight can induce oxidative and endoplasmic reticulum stress in the elderly and force the aged muscle to increase requirements from autophagy mechanisms. Impaired autophagy may be the main reason of myogenesis dysfunction during aging, which ultimately contributes to agerelated skeletal muscle loss [37]. Appropriate induction or accurate regulation of autophagic process and improved quality control of mitochondria through autophagy are required to maintain skeletal muscle mass [38].
2.8. Apoptosis
Myonuclear apoptosis is another molecular mechanism involved in muscle wasting and skeletal muscle atrophy [39,40]. In skeletal muscle, apoptosis exhibits unique characteristics in view of the multinuclear nature of myocytes. Activation of the apoptotic cascade leads to the removal of individual myonuclei and the relative portion of sarcoplasm. This pathway causes fiber atrophy rather than wholesale cell death. In addition, apoptotic signaling can stimulate muscle protein degradation through the activation of the UPS, resulting in fiber atrophy, independent of myonuclear removal [41]. Given that apoptotic signaling is required for protein degradation during muscle atrophy, muscle proteolysis and apoptosis are intimately connected [42]. There are two mitochondrial subpopulations with different bioenergy and structure in skeletal myofibers: subsarcolemmal mitochondria and intermyofibrillar mitochondria, which may differentially participate in the pathogenesis of sarcopenia and display different susceptibility to apoptotic stimuli [43].
2.9. Oxidative stress
During the aging process, the production of reactive oxygen species (ROS) may drastically increase because of altered respiratory chain function and impaired antioxidant cellular defences [44]. It has been reported that sarcopenia may be triggered by ROS which is involved in various cellular signaling processes. ROS can contribute to mitochondrial dysfunction and accelerate skeletal muscle damage and degeneration, which may consequently result in the occurrence of sarcopenia [45, 46]. Recent findings have revealed that muscle oxidative damage is associated with the mechanisms underlying excitation-contraction coupling. The unbalance of Ca2+ transport that is present in the sarcopenic muscle might be due to the altered oxidative state of those components involved in Ca2+ release and uptake [45].
3. CHF aggravates the adverse outcomes of sarcopenia
3.1. Falls
As a complication of sarcopenia, falls affect the physical and mental health of the elderly and increase the burden on families and society. The ilSIRENTE study assessed the association between sarcopenia and 2-year risk of falls in older population and indicated that participants with sarcopenia had a higher risk of incident falls compared with non sarcopenic subjects [47]. The underlying reason may be that sarcopenic individuals have poor stability and balance. Muscle fiber loss and atrophy with an inversion of type I to type II eventually result in decreased muscle mass and contractility [48]. CHF is usually accompanied by a variety of symptoms in elderly patients, such as dyspnea, frailty, decreased activity, poor cognitive function, and postural hypotension [49-51], which make older people more likely to fall. In addition, the production of ROS is significantly increased in CHF, which changes the structure of contractile proteins myosin and actin, eventually reducing muscle contractility [48]. Moreover, patients with CHF tend to have hypotension or need to take antihypertensive drugs. It has been reported that hypotension and use of antihypertensive medication are associated with an increased risk of falls among older adults [52, 53]. However, the recent REGARDS study including 5236 participants taking antihypertensive medication suggested that serious fall injuries among older adults are not related to blood pressure or number of antihypertensive medication classes, but to indicators of frailty [54]. In the treatment of CHF, we also need to pay attention to some drugs, such as digoxin and diuretics, which can potentially lead to falls [55].
3.2. Osteoporosis
Osteoporosis is a systemic bone disease characterized by low bone mass, bone microstructure damage, increased bone fragility, and easy fractures. Sarcopenia and osteoporosis are increasingly recognized as a “hazardous duet” and one is closely related to the other [56]. Growing evidence has shown that sarcopenia is an independent risk factor for low bone mineral density (BMD) and fracture, and the mechanisms are complex, including the effects of mechanical load on cell behaviors of bone and surrounding tissues, as well as the biological mechanisms of endocrine regulation between muscles and bones [57, 58]. A recent study involving 17,891 subjects with sarcopenia demonstrated that lean mass and grip strength were positively correlated with BMD and sarcopenia was related to low BMD and osteoporosis [59]. Another research revealed that the components of clinical sarcopenia were strongly associated with osteoporosis, and grip strength was the most significant measurement [60]. CHF can not only cause sarcopenia, but also make patients more prone to osteoporosis. For patients with CHF, long-term bed rest can lead to reduced outdoor activities, which has an adverse effect on the synthesis of vitamin D. In addition, long-term treatment with loop diuretics in CHF is associated with increased bone loss and fracture risk by inhibiting calcium reabsorption and increasing renal calcium excretion and bone turnover [61].
3.3. Frailty
Frailty is a clinical syndrome characterised by reduced physiological reserves affecting multiple organ systems and is related to increased risk of falls, fractures, hospitalisation and mortality [62]. The prevalence of sarcopenia and frailty in the elderly increases with age, and the two syndromes have similar performance, but each has its own characteristics. Sarcopenia is manifested as a progressive loss of muscle mass and muscle strength/function, while frailty is a state of increased vulnerability to stressors and reduced capacity to maintain homeostasis. There is overlap between these two conditions, especially in terms of the physical aspects of the frailty phenotype: low grip strength, gait speed and muscle mass [63]. Frailty often occurs in patients with CHF, and its prevalence varies from 18% to 54% depending on the study population and assessment methods [64]. The coexistence of these two geriatric syndromes may probably result from a common pathological pathway. A key feature of frailty is loss of muscle mass and strength that is not directly proportional to age. The mechanisms that lead to this enhanced catabolic state include derangements in the neurohormonal, metabolic, immunological, and musculoskeletal systems [65]. Peripheral loss of muscle tissue is a general finding in patients with CHF. The wasting syndrome in CHF affects all tissue compartments and is remarkably associated with neurohormonal and immunological abnormalities [66].
3.4. Cachexia
Cardiac cachexia is a syndrome involving progressive weight loss and alterations in body composition that carries a devastating prognosis in CHF [67]. Reduced muscle mass is an important characteristic of cachexia. Sarcopenia and cachexia share some similar mechanisms, including inflammation, insulin resistance, protein metabolism, and mitochondrial dysfunction [68, 69]. The main point to distinguish these two syndromes is weight loss, which is more predominant in cachexia rather than sarcopenia. Sarcopenia in CHF may eventually develop to cardiac cachexia, which is associated with poor prognosis [70, 71]. A clinical study showed that the prevalence of cachexia was 10.5% in stable CHF and was lower than previously anticipated due to the novel treatment strategies [72]. Cachexia and sarcopenia can overlap and be present in the same patient at the same time. Cachexia can be diagnosed with weighing scales only, whereas the detection of sarcopenia requires sophisticated body composition analysis [73].
3.5. Hospitalization and mortality
Sarcopenia, whether combined with CHF or alone, is a significant risk factor for hospitalization due to low muscle mass and strength. In the InCHIANTI study enrolling 538 sarcopenia participants, Cox regression analysis indicated that sarcopenia was associated with increased hospitalization and mortality after adjusting for potential confounders [74]. Okamura et al. retrospectively reviewed 1119 patients who underwent heart valve surgery and found that patients with sarcopenia had decreased long-term survival and increased major adverse cardiovascular events [75]. Muscle wasting is a frequent co-morbidity among patients with CHF. Sarcopenia and CHF can interact with each other and consequently contribute to reduced exercise capacity and adverse cardiovascular outcomes [76].
4. Treatments
4.1. Exercise
Currently, exercise is the most effective treatment for sarcopenia. Exercise training can exert beneficial effects on skeletal muscle through different mechanisms: mTORC1 activation, reduced oxidative stress, inhibition of inflammation, UPS inactivity, augmented mitochondrial biogenesis, increased IGF-1/myostatin ratio, and enhanced insulin sensitivity [77, 78]. Aerobic and resistance exercise is associated with reduced hospitalization and improved quality of life in patients with CHF [79]. Although sarcopenia is defined as a decrease in muscle mass and strength, resistance training may be the best physical exercise to prevent its occurrence and development, whereas aerobic exercise may be more suitable for cardiovascular fitness [80, 81]. The peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) is involved in the regulation of signaling pathways that control mitochondrial biogenesis and function responsible for muscle morphology and physiological function [82]. Physical exercise is a powerful stimulus to PGC-1α expression, which is crucial in protecting the muscle from several degradative and destructive processes, such as proteolysis, inflammation, oxidative damage, autophagy and apoptosis [83].
4.2. Nutrition
Besides exercise, nutrition should also be carefully evaluated, as a proper diet may be able to stimulate muscle anabolism and inhibit muscle catabolism. Protein is the most vital component for the elderly with regards to anabolic-catabolic balance, and excess protein intake can enhance not only muscle mass but also muscle function [84, 85]. Some essential amino acids and their metabolites, particularly the branched-chain amino acids, might exert beneficial effects in the treatment of CHF, such as enhancing protein synthesis and inhibiting proteolysis [86]. In addition, omega-3 polyunsaturated fatty acids might be an alternative therapeutic agent for sarcopenia due to their anti-inflammatory effects [87]. Moreover, vitamin and mineral supplements are also essential for sarcopenic patients with CHF. Vitamin E has antioxidant properties [88] and vitamin D positively affects muscle strength [89]. Mineral nutrients, especially calcium, magnesium and selenium, have been shown to prevent sarcopenia in observational studies [90].
4.3. Drug therapy
4.3.1. Standard CHF treatment
In the treatment of CHF, angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers, beta-blockers, and aldosterone antagonists can not only relieve clinical symptoms, but also attenuate ventricular remodeling and reduce long-term mortality [91]. ACE inhibitors have been shown to exert beneficial effects on sarcopenia via multiple biological pathways. They can improve mitochondrial function, increase IGF-1 levels, enhance insulin sensitivity, and promote glucose uptake in skeletal muscles [92]. However, a recent meta-analysis suggested that ACE inhibitors did not improve muscle strength and function in the elderly [93]. In addition, aldosterone antagonists such as spironolactone may delay the progression of sarcopenia by reducing skeletal myocyte apoptosis, improving vascular endothelial function and enhancing muscle contractility [94]. Furthermore, beta-blockers have been reported to increase body fat mass and improve cardiac function and exercise capacity in CHF patients [95]. For diastolic heart failure, there is currently no standard therapeutic strategy. Biomarkers of myocardial fibrosis, such as soluble source of tumorigenicity 2, growth differentiation factor 15, and galectin-3 play critical roles in the diagnosis and treatment of CHF with preserved ejection fraction [96]. Coronary heart disease is an important cause of CHF and statins are essential therapeutic drugs. Along with the main effect of cholesterol lowering, statins have some ancillary actions that may be relevant to sarcopenia. The potential mechanisms of statin-mediated muscle dysfunction involve IGF-1, inflammation, the UPS, apoptosis, and myostatin [97].
4.3.2. Hormonal treatment
4.3.2.1. Testosterone
The testosterone levels are significantly reduced in male patients with CHF [98]. Previous randomized controlled trials demonstrated that testosterone replacement therapy could improve muscle performance, exercise capacity, and insulin resistance in elderly patients with moderately severe CHF [99,100]. However, people worry about its various side effects, including increased risk of prostatic hyperplasia and cardiovascular events. Thus, selective androgen receptor modulator (SARM) is a theoretically better treatment strategy, as it has negligible side effects and equivalent function. Although SARMs have been shown to have positive effects on sarcopenia in some preclinical studies, large-scale trials are needed to confirm their efficacy and safety [101].
4.3.2.2. Growth hormone
Growth hormone replacement therapy can prevent sarcopenia by a dual mechanism: improvement of protein balance and antioxidant defense [102]. For patients with CHF, the clinical effectiveness of growth hormone has not been confirmed. Osterziel et al. conducted a randomized trial involving 50 CHF patients with dilated cardiomyopathy and found a significant increase in left ventricular mass in patients with growth hormone treatment, but this was not accompanied by an improvement in clinical status [103]. However, another placebo-controlled study demonstrated that recombinant human growth hormone had no significant effect on cardiac function, exercise capacity or neuroendocrine activation in patients with CHF [104].
4.3.2.3. Ghrelin
Ghrelin may be a promising therapeutic target for sarcopenia due to its positive effects on gastric motility, food intake, and growth hormone secretion. A previous study indicated that ghrelin treatment could effectively reduce the physical decline in sarcopenic mouse model through muscular enhancement and mitochondrial activation [105]. Moreover, a clinical study suggested that ghrelin administration could improve left ventricular function and exercise capacity, and increase muscle strength and lean body mass in patients with CHF [106]. However, some ghrelin analogues have been shown to improve body weight but have no effect on cardiac function in the rat model of CHF [107].
4.3.2.4. Insulin
Insulin can promote muscle protein synthesis by increasing muscle blood flow, amino acid delivery and availability [108]. A population-based study from Korea revealed that obese men with sarcopenia exhibited a significantly high risk of insulin resistance [109]. Additionally, patients with CHF are more prone to insulin resistance due to changes in neuroendocrine, inflammation, and inactivity [110]. Insulin supplementation may be beneficial for sarcopenic patients with CHF. However, a recent large-scale study demonstrated that insulin treatment might cause worse outcomes in patients with CHF and diabetes because of sodium retention and hypoglycemia [111]. Despite this, whether insulin use is associated with poor prognosis of CHF should be further investigated.
4.3.2.5. Thyroid hormone
Thyroid hormone is an important endocrine regulator with multiple biological functions. One major target of thyroid hormone is the skeletal muscle. They participate in a variety of biochemical events involved in the regulation of muscle mass and function [112]. It has been proved that overt and subclinical hyperthyroidism are related to the decline in muscle mass and strength [113]. In addition, elevated levels of thyroid-stimulating hormone are associated with increased hospitalization and mortality in patients with CHF [114]. However, the relationship between subclinical hypothyroidism and CHF is still controversial [115]. Thus, for CHF patients with sarcopenia, it is vital to maintain thyroid hormone at normal levels.
4.3.3. Myostatin inhibition
Myostatin is a member of the transforming growth factor beta family that is highly expressed in skeletal muscle [116]. It has been suggested that myostatin is a primary target of pharmacological interventions in muscle atrophy and wasting [117,118]. Therapeutic approaches have been taken to inhibit myostatin signaling both preclinically and clinically. Several myostatin inhibitors have been developed and used in clinical trials over the past decade. The results were disappointing and there was only limited effectiveness. However, a recent study indicated that bimagrumab may be a surprise. Bimagrumab treatment for 16 weeks was associated with increased muscle mass and strength in participants with sarcopenia and improved mobility in those with slow walking speed [119]. Further studies are needed to evaluate whether bimagrumab administration in the elderly with reduced skeletal muscle mass and impaired physical function could contribute to significant improvement in functional capacity and substantial prolongation of independence.
4.4. Assistive treatment
Elderly people who lack exercise or are unable to exercise due to a physical disability can undergo physical therapy, such as hydrotherapy, whole body vibration therapy, or functional electrical stimulation. Additionally, assistive technologies such as mobility aids, bathroom equipment, prostheses, communication devices, and specialized computer software and hardware also can prevent and/or treat muscle wasting, but the specific mechanisms and application conditions need to be further clarified [120].
Summary of some clinical trials included in this review.
| Study | Patients | Duration | Treatment | Main findings |
|---|---|---|---|---|
| Caminiti et al 99 | 70 elderly male patients with stable CHF, LVEF<40%, NYHA class II to III | 12 weeks | Intramuscular long-acting testosterone undecanoate (1000 mg) | Improvements in exercise capacity, muscle strength, glucose metabolism, and baroreflex sensitivity |
| Malkin et al 100 | 76 male patients with stable CHF | 12 months | A single 5 mg testosterone patch at night, replaced every 24 h | Improved functional capacity and symptoms |
| Osterziel et al 103 | 50 CHF patients with dilated cardiomyopathy, LVEF<45% | 12 weeks | Subcutaneous injection of rhGH (2 IU) daily | Increase in left-ventricular mass; no change in NYHA class, LVEF, or 6‑MWD |
| Isgaard et al 104 | 22 patients with CHF, LVEF<45%, NYHA class II to III | 3 months | Subcutaneous injection of rhGH (0.25 IU/kg/week) every evening | No effect on cardiac function, exercise capacity, or neuroendocrine activation |
| Nagaya et al 106 | 18 patients with stable CHF, LVEF<35% | 3 weeks | Intravenous ghrelin (2 μg/kg twice a day) | Increase in LVEF, 6‑MWD, pVO2, muscle strength, and lean body mass |
| Rooks et al 119 | 40 elderly people with sarcopenia | 16 weeks | Intravenous bimagrumab 30 mg/kg | Increased muscle mass and strength and improved mobility |
CHF = chronic heart failure; LVEF = left ventricular ejection fraction; NYHA = New York Heart Association; pVO2 = peak oxygen consumption; rhGH = recombinant human growth hormone; 6MWD = 6-min walk distance.
5. Conclusions
The diagnosis of sarcopenia has no uniform standard due to the influence of race, age, and gender. Currently, there is no particularly effective treatment strategy for this disease. CHF is a complex clinical syndrome with hundreds of years of history. Unfortunately, it is still a fortress that is difficult to overcome in the cardiovascular field. In recent years, the relationship between sarcopenia and CHF has not been fully understood. Patients with CHF are often accompanied by sarcopenia, which can cause a severe decline in quality of life and an increase in mortality. The diagnosis of sarcopenia depends on the evaluation of muscle mass, strength, and function. In addition, more specific biomarkers need to be identified for the diagnosis and staging of sarcopenia. Current drug development for the treatment of sarcopenia and CHF is still in the infancy stage, and the effectiveness and safety of the drugs are uncertain. Some representative clinical trials are presented in Table 1. In the future, the combination of physical exercise, nutritional supplements, and drug therapy may have the potential to counteract sarcopenia in CHF. In summary, it is necessary to screen for sarcopenia in patients with CHF. By establishing early evaluation methods and comprehensive treatment strategies, we can effectively delay the progression of sarcopenia in CHF and improve the life quality of patients.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (81770370) and Scientific Research Program for Young Talents of China National Nuclear Corporation (51001).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Cruz-Jentoft AJ, Landi F, Topinková E, Michel JP. Understanding sarcopenia as a geriatric syndrome. Curr Opin Clin Nutr Metab Care. 2010;13:1-7
2. Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T. et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16-31
3. Morley JE. Sarcopenia: diagnosis and treatment. J Nutr Health Aging. 2008;12:452-6
4. Ramani GV, Uber PA, Mehra MR. Chronic heart failure: contemporary diagnosis and management. Mayo Clin Proc. 2010;85:180-95
5. Narumi T, Watanabe T, Kadowaki S, Takahashi T, Yokoyama M, Kinoshita D. et al. Sarcopenia evaluated by fat-free mass index is an important prognostic factor in patients with chronic heart failure. Eur J Intern Med. 2015;26:118-22
6. Fülster S, Tacke M, Sandek A, Ebner N, Tschöpe C, Doehner W. et al. Muscle wasting in patients with chronic heart failure: results from the studies investigating co-morbidities aggravating heart failure (SICA-HF). Eur Heart J. 2013;34:512-9
7. Loncar G, Fülster S, von Haehling S, Popovic V. Metabolism and the heart: an overview of muscle, fat, and bone metabolism in heart failure. Int J Cardiol. 2013;162:77-85
8. Poehlman ET, Scheffers J, Gottlieb SS, Fisher ML, Vaitekevicius P. Increased resting metabolic rate in patients with congestive heart failure. Ann Intern Med. 1994;121:860-2
9. Aquilani R, Opasich C, Verri M, Boschi F, Febo O, Pasini E. et al. Is nutritional intake adequate in chronic heart failure patients? J Am Coll Cardiol. 2003;42:1218-23
10. İlhan B, Bahat G, Erdoğan T, Kılıç C, Karan MA. Anorexia Is Independently Associated with Decreased Muscle Mass and Strength in Community Dwelling Older Adults. J Nutr Health Aging. 2019;23:202-6
11. Sandek A, Doehner W, Anker SD, von Haehling S. Nutrition in heart failure: an update. Curr Opin Clin Nutr Metab Care. 2009;12:384-91
12. Hussain Z, Swindle J, Hauptman PJ. Digoxin use and digoxin toxicity in the post-DIG trial era. J Card Fail. 2006;12:343-6
13. Rolland Y, Czerwinski S, Abellan Van Kan G, Morley JE, Cesari M, Onder G. et al. Sarcopenia: its assessment, etiology, pathogenesis, consequences and future perspectives. J Nutr Health Aging. 2008;12:433-50
14. Drummond MJ, Dickinson JM, Fry CS, Walker DK, Gundermann DM, Reidy PT. et al. Bed rest impairs skeletal muscle amino acid transporter expression, mTORC1 signaling, and protein synthesis in response to essential amino acids in older adults. Am J Physiol Endocrinol Metab. 2012;302:E1113-22
15. Coker RH, Hays NP, Williams RH, Xu L, Wolfe RR, Evans WJ. Bed rest worsens impairments in fat and glucose metabolism in older, overweight adults. J Gerontol A Biol Sci Med Sci. 2014;69:363-70
16. Drummond MJ, Timmerman KL, Markofski MM, Walker DK, Dickinson JM, Jamaluddin M. et al. Short-term bed rest increases TLR4 and IL-6 expression in skeletal muscle of older adults. Am J Physiol Regul Integr Comp Physiol. 2013;305:R216-23
17. Dos Santos MR, Saitoh M, Ebner N, Valentova M, Konishi M, Ishida J. et al. Sarcopenia and Endothelial Function in Patients With Chronic Heart Failure: Results From the Studies Investigating Comorbidities Aggravating HF (SICA-HF). J Am Med Dir Asso. 2017;18:240-5
18. Kim J, Lang JA, Pilania N, Franke WD. Effects of blood flow restricted exercise training on muscular strength and blood flow in older adults. Exp Gerontol. 2017;99:127-32
19. Schaap LA, Pluijm SM, Deeg DJ, Harris TB, Kritchevsky SB, Newman AB. et al. Higher inflammatory marker levels in older persons: associations with 5-year change in muscle mass and muscle strength. J Gerontol A Biol Sci Med Sci. 2009;64:1183-9
20. Schrager MA, Metter EJ, Simonsick E, Ble A, Bandinelli S, Lauretani F. et al. Sarcopenic obesity and inflammation in the InCHIANTI study. J Appl Physiol. 2007;102:919-25
21. Onder G, Liperoti R, Russo A, Soldato M, Capoluongo E, Volpato S. et al. Body mass index, free insulin-like growth factor I, and physical function among older adults: results from the ilSIRENTE study. Am J Physiol Endocrinol Metab. 2006;291:E829-34
22. Dalla LL, Ravara B, Volterrani M, Gobbo V, Della Barbera M, Angelini A. et al. Beneficial effects of GH/IGF-1 on skeletal muscle atrophy and function in experimental heart failure. Am J Physiol Cell Physiol. 2004;286:C138-44
23. Bacurau AV, Jannig PR, de Moraes WM, Cunha TF, Medeiros A, Barberi L. et al. Akt/mTOR pathway contributes to skeletal muscle anti-atrophic effect of aerobic exercise training in heart failure mice. Int J Cardiol. 2016;214:137-47
24. Delafontaine P, Yoshida T. The renin-angiotensin system and the biology of skeletal muscle: mechanisms of muscle wasting in chronic disease states. Trans Am Clin Climatol Assoc. 2016;127:245-58
25. Josiak K, Jankowska EA, Piepoli MF, Banasiak W, Ponikowski P. Skeletal myopathy in patients with chronic heart failure: significance of anabolic-androgenic hormones. J Cachexia Sarcopenia Muscle. 2014;5:287-96
26. Jankowska EA, Biel B, Majda J, Szklarska A, Lopuszanska M, Medras M. et al. Anabolic deficiency in men with chronic heart failure prevalence and detrimental impact on survival. Circulation. 2006;114:1829-37
27. Storer TW, Magliano L, Woodhouse L, Lee ML, Dzekov C, Dzekov J. et al. Testosterone dose-dependently increases maximal voluntary strength and leg power, but does not affect fatigability or specific tension. J Clin Endocrinol Metab. 2003;88:1478-85
28. Ferrando AA, Sheffieldmoore M, Yeckel CW, Gilkison C, Jiang J, Achacosa A. et al. Testosterone administration to older men improves muscle function: molecular and physiological mechanisms. Am J Physiol Endocrinol Metab. 2002;282:E601-7
29. Estrada M, Espinosa A, Müller M, Jaimovich E. Testosterone stimulates intracellular calcium release and mitogen-activated protein kinases via a G protein-coupled receptor in skeletal muscle cells. Endocrinology. 2003;144:3586-97
30. LeBrasseur NK, Schelhorn TM, Bernardo BL, Cosgrove PG, Loria PM, Brown TA. Myostatin inhibition enhances the effects of exercise on performance and metabolic outcomes in aged mice. J Gerontol A Biol Sci Med Sci. 2009;64:940-8
31. Gruson D, Ahn SA, Ketelslegers JM, Rousseau MF. Increased plasma myostatin in heart failure. Eur J Heart Fail. 2011;13:734-6
32. Müller TD, Nogueiras R, Andermann ML, Andrews ZB, Anker SD, Argente J. et al. Ghrelin. Mol Metab. 2015;4:437-60
33. Saitoh M, Ishida J, Doehner W. Sarcopenia, cachexia, and muscle performance in heart failure Review update 2016. Int J Cardiol. 2017;238:5-11
34. Sandri M. Protein breakdown in muscle wasting: Role of autophagy-lysosome and ubiquitin-proteasome. Int J Biochem Cell Biol. 2013;45:2121-9
35. Gumucio JP, Mendias CL. Atrogin-1, MuRF-1, and sarcopenia. Endocrine. 2013;43:12-21
36. De Meyer GR, De Keulenaer GW, Martinet W. Role of autophagy in heart failure associated with aging. Heart Fail Rev. 2010;15:423-30
37. Potes Y, de Luxán-Delgado B, Rodriguez-González S, Guimarães MRM, Solano JJ, Fernández-Fernández M. et al. Overweight in elderly people induces impaired autophagy in skeletal muscle. Free Radic Biol Med. 2017;110:31-41
38. Fan J, Kou X, Jia S, Yang X, Yang Y, Chen N. Autophagy as a Potential Target for Sarcopenia. J Cell Physiol. 2016;231:1450-9
39. Calvani R, Joseph AM, Adhihetty PJ, Miccheli A, Bossola M, Leeuwenburgh C. et al. Mitochondrial pathways in sarcopenia of aging and disuse muscle atrophy. Biol Chem. 2013;394:393-414
40. Marzetti E, Calvani R, Bernabei R, Leeuwenburgh C. Apoptosis in skeletal myocytes: a potential target for interventions against sarcopenia and physical frailty - a mini-review. Gerontology. 2012;58:99-106
41. Du J, Wang X, Miereles C, Bailey JL, Debigare R, Zheng B. Activation of caspase-3 is an initial step triggering accelerated muscle proteolysis in catabolic conditions. J Clin Invest. 2004;113:115-23
42. Argilés JM, López-Soriano FJ, Busquets S. Apoptosis signalling is essential and precedes protein degradation in wasting skeletal muscle during catabolic conditions. Int J Biochem Cell Biol. 2008;40:1674-8
43. Marzetti E, Hwang JC, Lees HA, Wohlgemuth SE, Dupont-Versteegden EE, Carter CS. et al. Mitochondrial death effectors: relevance to sarcopenia and disuse muscle atrophy. Biochim Biophys Acta. 2010;1800:235-44
44. Viña J, Borras C, Abdelaziz K M, Garcia-Valles R, Gomez-Cabrera MC. The free radical theory of aging revisited: the cell signaling disruption theory of aging. Antioxid Redox Signal. 2013;19:779-87
45. Fulle S, Protasi F, Di Tano G, Pietrangelo T, Beltramin A, Boncompagni S. et al. The contribution of reactive oxygen species to sarcopenia and muscle ageing. Exp Gerontol. 2004;39:17-24
46. Bouzid MA, Filaire E, McCall A, Fabre C. Radical Oxygen Species, Exercise and Aging: An Update. Sports Med. 2015;45:1245-61
47. Landi F, Liperoti R, Russo A, Giovannini S, Tosato M, Capoluongo E. et al. Sarcopenia as a risk factor for falls in elderly individuals: results from the ilSIRENTE study. Clin Nutr. 2012;31:652-8
48. Thompson LV. Age-related muscle dysfunction. Exp Gerontol. 2009;44:106-11
49. Murad K, Kitzman DW. Frailty and multiple comorbidities in the elderly patient with heart failure: implications for management. Heart Fail Rev. 2012;17:581-8
50. Butrous H, Hummel SL. Heart Failure in Older Adults. Can J Cardiol. 2016;32:1140-7
51. Lazzarini V, Mentz RJ, Fiuzat M, Metra M, O'Connor CM. Heart failure in elderly patients: distinctive features and unresolved issues. Eur J Heart Fail. 2013;15:717-23
52. Klein D, Nagel G, Kleiner A, Ulmer H, Rehberger B, Concin H. et al. Blood pressure and falls in community-dwelling people aged 60 years and older in the VHM&PP cohort. BMC Geriatr. 2013;13:50
53. Tinetti ME, Han L, Lee DS, McAvay GJ, Peduzzi P, Gross CP. et al. Antihypertensive medications and serious fall injuries in a nationally representative sample of older adults. JAMA Intern Med. 2014;174:588-95
54. Bromfield SG, Ngameni CA, Colantonio LD, Bowling CB, Shimbo D, Reynolds K. et al. Blood pressure, antihypertensive polypharmacy, frailty, and risk for serious fall injuries among older treated adults with hypertension. Hypertension. 2017;70:259-66
55. Leipzig RM, Cumming RG, Tinetti ME. Drugs and falls in older people: a systematic review and meta-analysis: II. Cardiac and analgesic drugs. J Am Geriatr Soc. 1999;47:40-50
56. Crepaldi G, Maggi S. Sarcopenia and osteoporosis: A hazardous duet. J Endocrinol Invest. 2005;28:66-8
57. Yu HS, Kim JJ, Kim HW, Lewis MP, Wall I. Impact of mechanical stretch on the cell behaviors of bone and surrounding tissues. J Tissue Eng. 2016;7:2041731415618342
58. Digirolamo DJ, Clemens TL, Kousteni S. The skeleton as an endocrine organ. Nat Rev Rheumatol. 2012;8:674-83
59. He H, Liu Y, Tian Q, Papasian CJ, Hu T, Deng HW. Relationship of sarcopenia and body composition with osteoporosis. Osteoporos Int. 2016;27:473-82
60. Sjöblom S, Suuronen J, Rikkonen T, Honkanen R, Kröger H, Sirola J. Relationship between postmenopausal osteoporosis and the components of clinical sarcopenia. Maturitas. 2013;75:175-80
61. Mazziotti G, Canalis E, Giustina A. Drug-induced osteoporosis: mechanisms and clinical implications. Am J Med. 2010;123:877-84
62. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J. et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146-56
63. Dodds R, Sayer AA. Sarcopenia and frailty: new challenges for clinical practice. Clin Med (Lond). 2016;16:455-8
64. Jha SR, Ha HS, Hickman LD, Hannu M, Davidson PM, Macdonald PS. et al. Frailty in advanced heart failure: a systematic review. Heart Fail Rev. 2015;20:553-60
65. Joyce E. Frailty in Advanced Heart Failure. Heart Fail Clin. 2016;12:363-74
66. Anker SD, Ponikowski PP, Clark AL, Leyva F, Rauchhaus M, Kemp M. et al. Cytokines and neurohormones relating to body composition alterations in the wasting syndrome of chronic heart failure. Eur Heart J. 1999;20:683-93
67. von Haehling S, Lainscak M, Springer J, Anker SD. Cardiac cachexia: a systematic overview. Pharmacol Ther. 2009;121:227-52
68. Argilés JM, Busquets S, Stemmler B, López-Soriano FJ. Cachexia and sarcopenia: mechanisms and potential targets for intervention. Curr Opin Pharmacol. 2015;22:100-6
69. Ali S, Garcia JM. Sarcopenia, cachexia and aging: diagnosis, mechanisms and therapeutic options-a mini-review. Gerontology. 2014;60(4):294-305
70. Loncar G, Springer J, Anker M, Doehner W, Lainscak M. Cardiac cachexia: hic et nunc. J Cachexia Sarcopenia Muscle. 2016;7:246-60
71. Anker MS, von Haehling S, Springer J, Banach M, Anker SD. Highlights of the mechanistic and therapeutic cachexia and sarcopenia research 2010 to 2012 and their relevance for cardiology. Int J Cardiol. 2013;162:73-6
72. Christensen HM, Kistorp C, Schou M, Keller N, Zerahn B, Frystyk J. et al. Prevalence of cachexia in chronic heart failure and characteristics of body composition and metabolic status. Endocrine. 2013;43:626-34
73. von Haehling S, Ebner N, Dos Santos MR, Springer J, Anker SD. Muscle wasting and cachexia in heart failure: mechanisms and therapies. Nat Rev Cardiol. 2017;14:323-41
74. Bianchi L, Ferrucci L, Cherubini A, Maggio M, Bandinelli S, Savino E. et al. The Predictive Value of the EWGSOP Definition of Sarcopenia: Results From the InCHIANTI Study. J Gerontol A Biol Sci Med Sci. 2016;71:259-64
75. Okamura H, Kimura N, Tanno K, Mieno M, Matsumoto H, Yamaguchi A. et al. The impact of preoperative sarcopenia, defined based on psoas muscle area, on long-term outcomes of heart valve surgery. J Thorac Cardiovasc Surg. 2018 pii: S0022-5223(18)32032-4
76. Fülster S, Tacke M, Sandek A, Ebner N, Tschöpe C, Doehner W. et al. Muscle wasting in patients with chronic heart failure: results from the studies investigating co-morbidities aggravating heart failure (SICA-HF). Eur Heart J. 2013;34:512-9
77. Phu S, Boersma D, Duque G. Exercise and Sarcopenia. J Clin Densitom. 2015;18:488-92
78. Nascimento CM, Ingles M, Salvador-Pascual A, Cominetti MR, Gomez-Cabrera MC, Viña J. Sarcopenia, frailty and their prevention by exercise. Free Radic Biol Med. 2019;132:42-49
79. Piepoli MF, Conraads V, Corrà U, Dickstein K, Francis DP, Jaarsma T. et al. Exercise training in heart failure: from theory to practice. A consensus document of the Heart Failure Association and the European Association for Cardiovascular Prevention and Rehabilitation. Eur J Heart Fail. 2011;13:347-57
80. Peterson MD, Rhea MR, Sen A, Gordon PM. Resistance exercise for muscular strength in older adults: a meta-analysis. Ageing Res Rev. 2010;9:226-37
81. Fernhall B. Long-term aerobic exercise maintains peak VO(2), improves quality of life, and reduces hospitalisations and mortality in patients with heart failure. J Physiother. 2013;59:56
82. Ji LL, Kang C. Role of PGC-1α in sarcopenia: etiology and potential intervention - a mini-review. Gerontology. 2015;61:139-48
83. Lira VA, Benton CR, Yan Z, Bonen A. PGC-1alpha regulation by exercise training and its influences on muscle function and insulin sensitivity. Am J Physiol Endocrinol Metab. 2010;299:E145-61
84. Beasley JM, Shikany JM, Thomson CA. The role of dietary protein intake in the prevention of sarcopenia of aging. Nutr Clin Pract. 2013;28:684-90
85. Naseeb MA, Volpe SL. Protein and exercise in the prevention of sarcopenia and aging. Nutr Res. 2017;40:1-20
86. Von Haehling S, Doehner W, Anker SD. Nutrition, metabolism, and the complex pathophysiology of cachexia in chronic heart failure. Cardiovasc Res. 2007;73:298-309
87. Dupont J, Dedeyne L, Dalle S, Koppo K, Gielen E. The role of omega-3 in the prevention and treatment of sarcopenia. Aging Clin Exp Res. 2019 doi: 10.1007/s40520-019-01146-1
88. Khor SC, Abdul Karim N, Ngah WZ, Yusof YA, Makpol S. Vitamin E in sarcopenia: current evidences on its role in prevention and treatment. Oxid Med Cell Longev. 2014;2014:914853
89. Lappe JM, Binkley N. Vitamin D and Sarcopenia/Falls. J Clin Densitom. 2015;18:478-82
90. Van Dronkelaar C, van Velzen A, Abdelrazek M, van der Steen A, Weijs PJM, Tieland M. Minerals and Sarcopenia; The Role of Calcium, Iron, Magnesium, Phosphorus, Potassium, Selenium, Sodium, and Zinc on Muscle Mass, Muscle Strength, and Physical Performance in Older Adults: A Systematic Review. J Am Med Dir Assoc. 2018;19:6-11
91. Raj L, Adhyaru B. An evidence-based review of recent advances in therapy for heart failure with reduced ejection fraction (HFrEF). Postgrad Med J. 2016;92:726-34
92. Springer J, von Haehling S. ACE Inhibitors and Sarcopenia: Covering All the BASEs? Drugs Aging. 2016;33:839-40
93. Zhou LS, Xu LJ, Wang XQ, Huang YH, Xiao Q. Effect of Angiotensin-Converting Enzyme Inhibitors on Physical Function in Elderly Subjects: A Systematic Review and Meta-Analysis. Drugs Aging. 2015;32:727-35
94. Burton L A, Mcmurdo ME, Struthers A D. Mineralocorticoid antagonism: a novel way to treat sarcopenia and physical impairment in older people? Clin Endocrinol (Oxf). 2011;75:725-9
95. Lainscak M, Keber I, Anker SD. Body composition changes in patients with systolic heart failure treated with beta blockers: a pilot study. Int J Cardiol. 2006;106:319-22
96. Michalska-Kasiczak M, Bielecka-Dabrowa A, von Haehling S, Anker SD, Rysz J, Banach M. Biomarkers, myocardial fibrosis and co-morbidities in heart failure with preserved ejection fraction: an overview. Arch Med Sci. 2018;14:890-909
97. Bielecka-Dabrowa A, Fabis J, Mikhailidis DP, von Haehling S, Sahebkar A, Rysz J. et al. Prosarcopenic effects of statins may limit their effectiveness in patients with heart failure. Trends Pharmacol Sci. 2018;39:331-53
98. Jankowska EA, Filippatos G, Ponikowska B, Borodulin-Nadzieja L, Anker SD, Banasiak W. et al. Reduction in circulating testosterone relates to exercise capacity in men with chronic heart failure. J Card Fail. 2009;15:442-50
99. Caminiti G, Volterrani M, Iellamo F, Marazzi G, Massaro R, Miceli M. et al. Effect of long-acting testosterone treatment on functional exercise capacity, skeletal muscle performance, insulin resistance, and baroreflex sensitivity in elderly patients with chronic heart failure a double-blind, placebo-controlled, randomized study. J Am Coll Cardiol. 2009;54:919-27
100. Malkin CJ, Pugh PJ, West JN, van Beek EJ, Jones TH, Channer KS. Testosterone therapy in men with moderate severity heart failure: a double-blind randomized placebo controlled trial. Eur Heart J. 2006;27:57-64
101. Morley JE. Pharmacologic Options for the Treatment of Sarcopenia. Calcif Tissue Int. 2016;98:319-33
102. Brioche T, Kireev RA, Cuesta S, Gratas-Delamarche A, Tresguerres JA, Gomez-Cabrera MC. et al. Growth hormone replacement therapy prevents sarcopenia by a dual mechanism: improvement of protein balance and of antioxidant defenses. J Gerontol A Biol Sci Med Sci. 2014;69:1186-98
103. Osterziel KJ, Strohm O, Schuler J, Friedrich M, Hänlein D, Willenbrock R. et al. Randomised, double-blind, placebo-controlled trial of human recombinant growth hormone in patients with chronic heart failure due to dilated cardiomyopathy. Lancet. 1998;351:1233-7
104. Isgaard J, Bergh CH, Caidahl K, Lomsky M, Hjalmarson A, Bengtsson BA. A placebo-controlled study of growth hormone in patients with congestive heart failure. Eur Heart J. 1998;19:1704-11
105. Tamaki M, Miyashita K, Hagiwara A, Wakino S, Inoue H, Fujii K. et al. Ghrelin treatment improves physical decline in sarcopenia model mice through muscular enhancement and mitochondrial activation. Endocr J. 2017;64:S47-S51
106. Nagaya N, Moriya J, Yasumura Y, Uematsu M, Ono F, Shimizu W. et al. Effects of ghrelin administration on left ventricular function, exercise capacity, and muscle wasting in patients with chronic heart failure. Circulation. 2004;110:3674-9
107. Palus S, Schur R, Akashi YJ, Bockmeyer B, Datta R, Halem H. et al. Ghrelin and its analogues, BIM-28131 and BIM-28125, improve body weight and regulate the expression of MuRF-1 and MAFbx in a rat heart failure model. PLoS One. 2011;6:e26865
108. Fujita S, Rasmussen BB, Cadenas JG, Grady JJ, Volpi E. Effect of insulin on human skeletal muscle protein synthesis is modulated by insulin-induced changes in muscle blood flow and amino acid availability. Am J Physiol Endocrinol Metab. 2006;291:E745-54
109. Kwon SS, Lee SG, Lee YH, Lim JB, Kim JH. Homeostasis model assessment of insulin resistance in a general adult population in Korea: additive association of sarcopenia and obesity with insulin resistance. Clin Endocrinol (Oxf). 2017;86:44-51
110. Swan JW, Anker SD, Walton C, Godsland IF, Clark AL, Leyva F. et al. Insulin Resistance in Chronic Heart Failure: Relation to Severity and Etiology of Heart Failure. J Am Coll Cardiol. 1997;30:527-32
111. Cosmi F, Shen L, Magnoli M, Abraham WT, Anand IS, Cleland JG. et al. Treatment with insulin is associated with worse outcome in patients with chronic heart failure and diabetes. Eur J Heart Fail. 2018;20:888-95
112. Bloise FF, Cordeiro A, Ortiga-Carvalho TM. Role of thyroid hormone in skeletal muscle physiology. J Endocrinol. 2018;236:R57-R68
113. Brennan MD, Powell C, Kaufman KR, Sun PC, Bahn RS, Nair KS. The impact of overt and subclinical hyperthyroidism on skeletal muscle. Thyroid. 2006;16:375-80
114. Chen S, Shauer A, Zwas DR, Lotan C, Keren A, Gotsman I. The effect of thyroid function on clinical outcome in patients with heart failure. Eur J Heart Fail. 2014;16:217-26
115. Bielecka-Dabrowa A, Godoy B, Suzuki T, Banach M, von Haehling S. Subclinical hypothyroidism and the development of heart failure: an overview of risk and effects on cardiac function. Clin Res Cardiol. 2019;108:225-33
116. Elkina Y, von Haehling S, Anker SD, Springer J. The role of myostatin in muscle wasting: an overview. J Cachexia Sarcopenia Muscle. 2011;2:143-51
117. Bogdanovich S, Krag TO, Barton ER, Morris LD, Whittemore LA, Ahima RS. et al. Functional improvement of dystrophic muscle by myostatin blockade. Nature. 2002;420:418-21
118. von Haehling S, Lainscak M, Springer J, Anker SD. Cardiac cachexia: a systematic overview. Pharmacol Ther. 2009;121:227-52
119. Rooks D, Praestgaard J, Hariry S, Laurent D, Petricoul O, Perry RG. et al. Treatment of Sarcopenia with Bimagrumab: Results from a Phase II, Randomized, Controlled, Proof-of-Concept Study. J Am Geriatr Soc. 2017;65:1988-95
120. Scott RA, Callisaya ML, Duque G, Ebeling PR, Scott D. Assistive technologies to overcome sarcopenia in ageing. Maturitas. 2018;112:78-84
Author contact
![]() Corresponding author: Xiang Zhou, Department of Cardiology, The Second Affiliated Hospital of Soochow University, No. 1055 Sanxiang Road, Suzhou, 215004, P.R. China. Tel: +86 512 67784079; Fax: +86 512 68284303; E-mail: zhou-xiangedu.cn
Corresponding author: Xiang Zhou, Department of Cardiology, The Second Affiliated Hospital of Soochow University, No. 1055 Sanxiang Road, Suzhou, 215004, P.R. China. Tel: +86 512 67784079; Fax: +86 512 68284303; E-mail: zhou-xiangedu.cn
 Global reach, higher impact
Global reach, higher impact