13.3
Impact Factor
Theranostics 2019; 9(12):3653-3658. doi:10.7150/thno.31032 This issue Cite
Research Paper
Prospective non-invasive evaluation of CXCR4 expression for the diagnosis of MALT lymphoma using [68Ga]Ga-Pentixafor-PET/MRI
1. Department of Biomedical Imaging and Image-guided Therapy, Division of Nuclear Medicine, Medical University of Vienna, Austria;
2. Ludwig Boltzmann Institute Applied Diagnostics, Vienna, Austria.
3. Scintomics GmbH, Garching, Germany.
4. Department of Internal Medicine I, Division of Oncology, Medical University of Vienna, Austria.
5. Department of Biomedical Imaging and Image-guided Therapy, Division of General and Paediatric Radiology, Medical University of Vienna, Austria;
6. Christian-Doppler Laboratory for Applied Metabolomics, Division of Nuclear Medicine, Medical University of Vienna, Austria.
7. Department of Radiology, Memorial Sloan Kettering Cancer Center, New York, NY.
Received 2018-10-28; Accepted 2019-3-28; Published 2019-5-27
Abstract
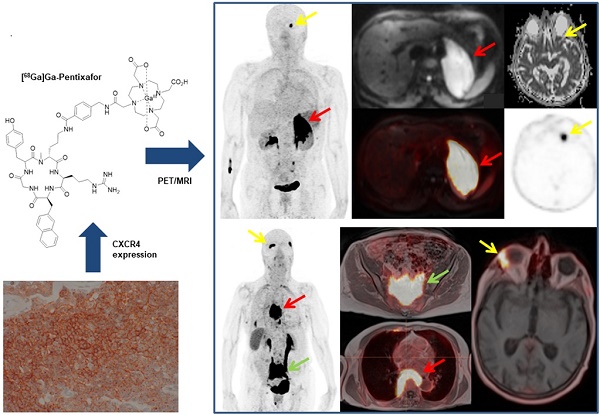
MALT lymphomas express the chemokine receptor CXCR4 on a regular basis, and [68Ga]Ga-Pentixafor-PET has been shown to quantify CXCR4 expression non-invasively. We, therefore, aimed to evaluate [68Ga]Ga-Pentixafor-PET/MRI for the non-invasive assessment of MALT lymphomas.
Methods: We included 36 MALT lymphoma patients, who had not undergone previous systemic or radiation therapy, in our prospective, IRB-approved, proof-of-concept study. Involved anatomic regions were the orbit (n=14), stomach (n=10), lungs (n=5), and other sites (soft-tissues n=3; adrenal gland, tonsils, parotid gland, and urinary bladder n=1, respectively). MRI sequences included an axial 2-point Dixon T1 VIBE SPAIR 3D sequence for PET attenuation correction; a coronal T2 HASTE sequence; and an axial echo-planar imaging SPAIR-based diffusion-weighted sequence (DWI) obtained during free-breathing (b-values, 50 and 800), with corresponding ADC (apparent diffusion coefficient) maps.
Results: In 33/36 patients, there were MALT lymphomas with an increased uptake of [68Ga]Ga-Pentixafor; all current lymphoma manifestations showed an increased uptake and, accordingly, were positive on the PET/MRI. The remaining three patients had undergone surgery for their orbital MALT lymphomas prior to PET/MRI. Mean SUVmax was 8.6 ± 4.7, mean SUVmean was 4.7 ± 1.8, and mean SUVpeak was 8.0 ± 4.2. The mean SUVmax of the liver was 1.8, and the mean tumor-to-liver ratio was 2.9 ± 2.0. There were no significant differences in SUVmax (P=0.22), SUVmean (P=0.53), SUVpeak (P=0.29), or SUVt/l (P=0.92) between the four anatomic regions (orbit, stomach, lungs, other). The mean tumor volume was 146 ± 499.
Conclusions: Our results thus indicate that [68Ga]Ga-Pentixafor-PET is feasible for the assessment of MALT lymphomas, with a good tumor-to-background ratio in terms of radiotracer uptake.
Keywords: MALT lymphoma, PET, CXCR4, [68Ga]-Pentixafor, PET/MRI
Introduction
The staging or localization of extra-nodal, marginal zone, B-cell lymphoma of the mucosa-associated lymphoid tissue (MALT) lymphoma is an imaging challenge. Generally, computed tomography of the chest, abdomen, and pelvis, in combination with physical examination, is recommended, but this is a strategy that suffers from limited diagnostic accuracy [1]. MALT lymphomas frequently do not present with an elevated glycolysis, which hampers the use of 2-deoxy-2-[18F]fluoro-D-glucose-positron emission tomography ([18F]FDG-PET) [2]. Most MALT lymphomas, which can arise in almost every part of the body, lack specific symptoms; gastric MALT lymphomas display only nonspecific upper abdominal / gastrointestinal symptoms, and thus, incidental diagnosis is often based on endoscopic biopsies [1, 3]. As up to 25% of all gastric and 46% of all extra-gastric MALT lymphomas present with multi-organ involvement, and the presence of localized versus multi-organ involvement crucially influences treatment, a reliable method for whole-body staging is required [1, 3, 4]. Magnetic resonance imaging (MRI), especially with diffusion-weighted imaging, has emerged as a promising imaging tool [2]. However, the value of whole-body MRI for diagnosis and staging has yet to be evaluated and has not been recommended in the guidelines as yet [1, 3, 5].
Recently, non-invasive quantification and imaging of C-X-C chemokine receptor type 4 (CXCR4) has emerged for hematological malignancies [6]. CXCR4 is expressed in a variety of blood cells; its activation with stroma-derived factor 1 (SDF-1) activates the MAP kinase and PI3 kinase pathway [7]. CXCR4 is overexpressed in many types of solid cancers and is associated with proliferation, metastasis, and angiogenesis, which results in a poor prognosis [8]. High levels of CXCR4 expression have also been reported in hematopoietic malignancies, such as mantle cell lymphoma and MALT lymphoma, [9] and have also been correlated with a poor prognosis [10]. Consequently, drugs that act as antagonists on CXCR4 have been evaluated as new therapeutic tools [11, 12]. [68Ga]Ga-Pentixafor is a radiopharmaceutical for PET that binds with high specificity, selectivity, and has excellent clearance characteristics [13]. All of which make non-invasive [68Ga]Ga-Pentixafor-PET a promising diagnostic tool for malignancies associated with CXCR4 overexpression.
In the present study, it was our aim to evaluate [68Ga]Ga-Pentixafor-PET/MRI for the assessment of MALT lymphomas.
Results and Discussion
Thirty-six MALT lymphoma patients (median age, 62; range, 35-87 years; 19 female), who had not undergone previous systemic or radiation therapy, were included. MALT lymphomas were located in the orbit (n=14), the stomach (n=10), the lungs (n=5), or other sites (n=7; soft-tissues n=3; adrenal gland, tonsils, parotid gland, and urinary bladder; n=1, respectively) (Table 1). All but three patients demonstrated MALT lymphoma with markedly increased [68Ga]Ga-Pentixafor uptake, as quantified by standardized uptake values (mean SUVmax, 8.6; range, 3.1 to 24.4). The three PET-negative patients showed MALT lymphoma of the orbit— in these cases, the initial diagnosis was made by surgical resection, prior to the PET/MRI examination. Since, in these cases, neither diffusion-weighted imaging (DWI) nor follow-up sonography revealed residual MALT lymphoma, [68Ga]Ga-Pentixafor-PET was rated as true-negative. No additional lesions that were rated as negative in the PET/MRI were detected in other investigations.
There were no significant differences in SUVmax (P=0.22), SUVmean (P=0.53), SUVt/l (P=0.92), SUVpeak (P=0.29), or metabolic tumor volume (MTV) (P=0.74) between anatomic locations of the MALT lymphomas described above. The mean SUVmax of gastric MALT lymphoma was not significantly different from that of non-gastric MALT lymphoma (7.1 versus 9.2; P=0.49). The same was true with regard to mean SUVt/l (2.8 versus 3.0; P=0.92) and MTV, defined as a volume of interest (VOI) using an SUV threshold of 2.5 (295 versus 75ml; P=0.52). In six patients, additional MALT lymphoma manifestations were detected by [68Ga]Ga-Pentixafor PET. In one patient with an adrenal gland MALT lymphoma, an additional orbital involvement was diagnosed (Figure 1); in one patient with gastric MALT lymphoma, involvement of an adjacent mesenteric lymph node with high [68Ga]Ga-Pentixafor uptake (SUVmax 7.4; Figure 2) was observed; in two patients with orbital and lung MALT lymphoma, respectively, and which were presumed to be unilateral, bilateral involvement was observed; in one patient with MALT lymphoma of the orbit, multifocal involvement of the upper cervical, paravertebral, pericardial, and pre-sacral soft tissues was diagnosed; and in one patient with cutaneous/subcutaneous MALT lymphoma, multifocal involvement was diagnosed. All of these additional MALT lymphoma manifestations showed an increased [68Ga]Ga-Pentixafor uptake. In 22/36 patients, no enlarged cervical lymph nodes showed an uptake on [68Ga]Ga-Pentixafor PET. The mean SUVmax was 6.5, SUVmean was 4.1, SUVpeak was 6.0, and volume was 3.6 ml. Compared to the uptake values of MALT lymphoma, the SUVmax and SUVpeak of the cervical lymph nodes was significantly lower compared to those of MALT lymphoma (SUVmax 6.5 vs. 8.7; P<0.05; SUVmean 4.1 vs. 4.7; P=0.1; SUVpeak 6.0 vs. 8.0; P<0.05). This increased uptake might be attributable to activated leukocytes, as CXCR4 is one of the main chemokine receptors involved in B cell homing to secondary lymphoid tissue [10].Consequently, in one patient the increased lymph node uptake did not represent MALT lymphoma involvement at histology, but, rather, inflammatory hyperplasia.
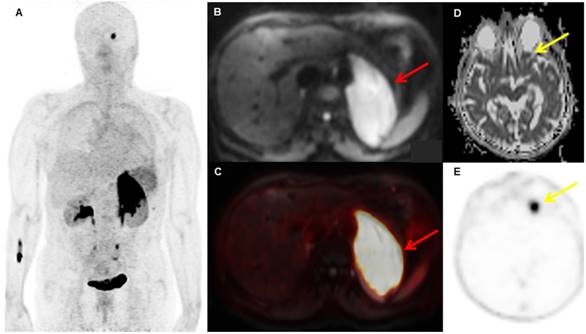
A patient with biopsy-proven MALT lymphoma of the left adrenal gland. The adrenal MALT lymphoma shows high [68Ga]Ga-Pentixafor uptake (A, SUVmax 25.4), and is also well-visualized on the DWI b800 image (B, red arrow, C, fused PET/MRI). In addition, an area of increased uptake on PET is visible in the left orbit (E, yellow arrow), which was initially missed on MRI, but, in retrospect, showed restricted diffusion on the ADC map upon consensus reading (D, yellow arrow); this orbital lesion also proved to be MALT lymphoma at histology.
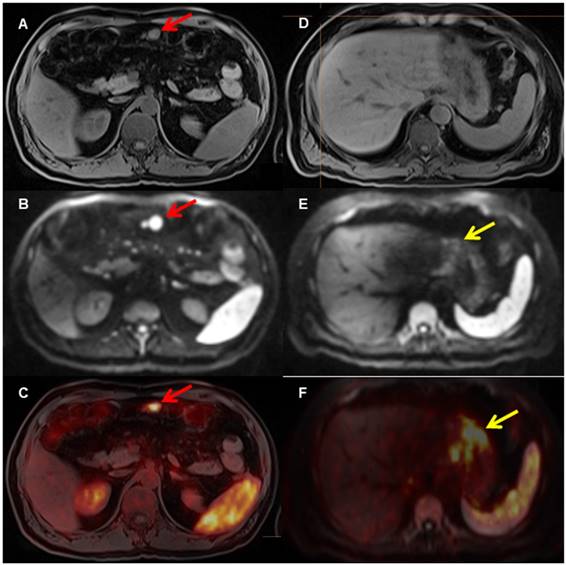
A patient with biopsy-proven gastric MALT lymphoma. The [68Ga]Pentixafor PET shows several areas in the stomach with increased [68Ga]Ga-Pentixafor uptake (F, yellow arrow on fused PET/MRI) and restricted diffusion on DWI (E, yellow arrow, D T1 VIBE Dixon), indicating the MALT lymphoma. In addition, there is evidence of an enlarged mesenteric lymph node (A, T1 with restricted diffusion on DWI b_800 (B, red arrow) and markedly increased uptake on PET (C, red arrow on fused PET/MRI), consistent with lymph node involvement.
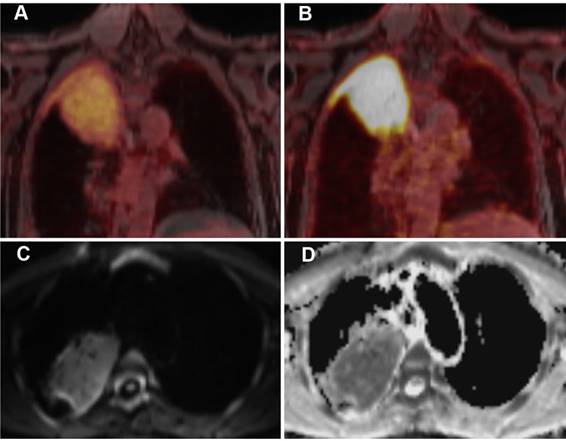
A 63-year-old patient with MALT lymphoma in the upper lobe / apex of the right lung and adjacent pleura. The lymphoma presents as a large consolidation with strong diffusion restriction (high signal on the DWI image (C) and low signal on the corresponding ADC map (D)). On PET, the lymphoma shows only a moderate uptake of [18F]FDG (A), indicating moderately increased glucose metabolism, but a strong uptake of [68Ga]Ga-Pentixafor, suggesting a high CXCR4 expression (B).
Overview of the uptake values and volumes of MALT lymphoma in all patients. In addition, the respective data is given with regard to the organ involved by the MALT lymphoma.
| Type | Age | SUVmax | SUVmean | SUVpeak | Volume | SUV liver | SUV tumor/liver |
|---|---|---|---|---|---|---|---|
| All (n=36) | 62 ±13 [35-87] | 8.6±4.7 [3.1-24.4] | 4.7±1.8 [2.8-10.2] | 8.0±4.2 [3.4-22.7] | 146±499 [0.4-2777] | 3.4±2.2[1.6-12.8] | 2.9±2.0 [0.9-10.9] |
| Stomach (n=10) | 60 | 7.1 | 4.0 | 6.2 | 295 | 2.8 | 2.8 |
| Orbit (n=14) | 63 | 8.1 | 4.9 | 7.8 | 58 | 3.0 | 3.1 |
| Lung (n=5) | 61 | 12.4 | 5.4 | 11.0 | 68 | 5.1 | 3.4 |
| Other (n=7) | 62 | 8.6 | 4.7 | 8.0 | 146 | 3.4 | 2.9 |
The above findings demonstrate the potential of [68Ga]Ga-Pentixafor-PET/MRI in the assessment of MALT lymphoma. All MALT lymphomas included showed a high uptake of [68Ga]Ga-Pentixafor, with an excellent tumor-to-background ratio (Figure 3). The present results are consistent with the recently reported high level of CXCR4 expression in MALT lymphoma [9]. Interestingly, uptake (in terms of SUVs) differed considerably between patients, and thus, we speculate that the degree of [68Ga]Ga-Pentixafor uptake may possibly provide prognostic information, as a correlation between proliferation (as measured with Ki-67) and the expression of CXCR4 in MALT lymphoma has been previously described [9]. Therefore, [68Ga]Ga-Pentixafor might provide not only a high diagnostic accuracy, but also enable non-invasive prognostic stratification based on the uptake.
The diagnostic value of [68Ga]Ga-Pentixafor-PET/MRI is underlined by the fact that it revealed previously unknown, additional lymphoma manifestations in 17% of patients, compared to MRI, which may affect staging and may have therapeutic implications. Nevertheless, the increased expression of CXCR4 in uninvolved (i.e., non-lymphomatous) cervical lymph nodes has to be considered, which was most probably due to inflammatory changes; after all, it has been shown that CXCR4 is expressed on T and B lymphocytes, macrophages, monocytes, neutrophils, and eosinophils [14, 15]. A possible limitation for future applications might be the current shortage of available approved generators and rather complex logistics with the need for a separate synthesis for almost every patient.
Notably, [68Ga]Ga-Pentixafor might, in the future, gain relevance as a companion diagnostic compound. CXCR4 antagonists are currently being tested in early-phase studies, e.g., in acute myeloid leukemia [16]. As a companion diagnostic tool, [68Ga]Ga-Pentixafor-PET could reveal the uniform expression of CXCR4 in all lymphoma manifestations in terms of enhanced patient selection. An initial study reported the use of a therapeutic analogue of Pentixafor, Pentixather, labeled with β-emitting Lutetium-177, a commonly used therapeutic radionuclide [17]. Future studies will evaluate whether this therapeutic approach could also be useful in patients with MALT lymphoma.
In conclusion, this first study confirmed the effective value of [68Ga]Ga-Pentixafor PET/MRI in the assessment of MALT lymphoma, with an excellent tumor-to-background contrast.
Methods
We prospectively included patients with newly diagnosed, histology-proven MALT lymphoma in our proof-of-concept study, which was approved by the local ethics committee; all patients gave written, informed consent. Exclusion criteria were: previous systemic therapy; age <18 years; pregnancy; breastfeeding; and known contraindications to MRI. [68Ga]Ga-Pentixafor production was carried out, as previously described, in a fully automated manner using a Scintomics GRP module [13]. Sixty minutes after the intravenous administration of a median of 172 MBq of [68Ga]Ga-Pentixafor, PET/MRI, using an integrated scanner (Biograph mMR; Siemens, Erlangen, Germany), was performed, with 5 min per bed position and the point-spread function-based reconstruction algorithm HD-PET for PET; an axial 2-point Dixon T1 VIBE SPAIR 3D sequence for PET attenuation correction; a coronal T2 HASTE sequence; and an axial echo-planar imaging SPAIR-based diffusion-weighted sequence (DWI) obtained during free-breathing (b-values, 50 and 800), with corresponding ADC (apparent diffusion coefficient) maps.
Two senior, board-certified readers (one radiologist and one nuclear medicine physician) evaluated, in consensus, the co-registered PET and MRI using a Syngo workstation (Siemens, Erlangen, Germany) and a Hermes Hybrid Viewer (Hermes Medical solutions, Stockholm, Sweden) to draw semi-automated volumes of interest (VOI) using an SUV threshold of 2.5. The obtained volume was defined as the metabolic tumor volume. On PET, nodal and extranodal regions were rated as positive for lymphoma when there was at least one focal (or, for bone marrow, diffuse) area of increased tracer accumulation, relative to the surrounding tissue or mediastinal blood pool activity. For MRI (with DWI), we used previously published criteria for extranodal and nodal (>1.5 cm) lymphoma manifestations [2]. In case of PET/MRI findings not previously confirmed by endoscopy and biopsy, additional biopsies and histological verifications had to be performed to achieve a reliable reference standard. Independent-sample t-tests were used for group comparisons (significance level, P<0.05).
Acknowledgements
No funding was received for this study. We would like to thank the staff of the PET/MRI for their help in conducting this study. We thank Prof. Amelie Lupp from the Institute of Pharmacology and Toxicology, Jena University Hospital, for providing us with CXCR4 immunostaining.
Author contributions
A.H., M.Ma., and M.R. designed the study; A.H. wrote the primary and subsequent versions of the manuscript; A.L., M.R., and M.Har. recruited patients; M.Mi., W.W., S.P., H.W., and S.K. helped to develop and establish [68Ga]Ga-Pentixafor synthesis and enabled supply. M.Hac., M.Mi., W.W., H.W., and S.K. aided in refining the study and helped prepare the manuscript; all coauthors have reviewed and edited the manuscript.
Competing Interests
H.W. is a founder and shareholder of Scintomics. S.K. is CEO of Scintomics. M.Ma. received research grants and honoraria for presentations from Siemens Healthineers. A.R.H. received research grants from Siemens Healthineers. The other authors declare no competing financial interests.
References
1. Zucca E, C Copie-Bergman U Ricardi, C Thieblemont M Raderer, M Ladetto et al. Gastric marginal zone lymphoma of MALT type: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl 6):144-8
2. Mayerhoefer ME, G Karanikas K Kletter, H Prosch B Kiesewetter, C Skrabs et al. Evaluation of diffusion-weighted MRI for pretherapeutic assessment and staging of lymphoma: results of a prospective study in 140 patients. Clin Cancer Res. 2014;20:2984-93
3. Ruskone-Fourmestraux A, W Fischbach BM Aleman, H Boot MQ Du, F Megraud et al. EGILS consensus report. Gastric extranodal marginal zone B-cell lymphoma of MALT. Gut. 2011;60:747-58
4. Raderer M, S Wohrer B Streubel, M Troch K Turetschek, U Jager et al. Assessment of disease dissemination in gastric compared with extragastric mucosa-associated lymphoid tissue lymphoma using extensive staging: a single-center experience. J Clin Oncol. 2006;24:3136-41
5. Dreyling M, C Thieblemont A Gallamini, L Arcaini E Campo, O Hermine et al. ESMO Consensus conferences: guidelines on malignant lymphoma. part 2: marginal zone lymphoma, mantle cell lymphoma, peripheral T-cell lymphoma. Ann Oncol. 2013;24:857-77
6. Philipp-Abbrederis K, K Herrmann S Knop, M Schottelius M Eiber, K Luckerath et al. In vivo molecular imaging of chemokine receptor CXCR4 expression in patients with advanced multiple myeloma. EMBO Mol Med. 2015;7:477-87
7. Jacobson O, ID Weiss. CXCR4 chemokine receptor overview: biology, pathology and applications in imaging and therapy. Theranostics. 2013;3:1-2
8. Zhao H, L Guo H Zhao, J Zhao H Weng, B Zhao. CXCR4 over-expression and survival in cancer: a system review and meta-analysis. Oncotarget. 2015;6:5022-40
9. Stollberg S, D Kammerer E Neubauer, S Schulz I Simonitsch-Klupp, B Kiesewetter et al. Differential somatostatin and CXCR4 chemokine receptor expression in MALT-type lymphoma of gastric and extragastric origin. J Cancer Res Clin Oncol. 2016;142:2239-47
10. Durig J, U Schmucker U Duhrsen. Differential expression of chemokine receptors in B cell malignancies. Leukemia. 2001;15:752-6
11. Cho BS, Z Zeng H Mu, Z Wang S Konoplev, T McQueen et al. Antileukemia activity of the novel peptidic CXCR4 antagonist LY2510924 as monotherapy and in combination with chemotherapy. Blood. 2015;126:222-32
12. Uy GL, MP Rettig IH Motabi, K McFarland KM Trinkaus, LM Hladnik et al. A phase 1/2 study of chemosensitization with the CXCR4 antagonist plerixafor in relapsed or refractory acute myeloid leukemia. Blood. 2012;119:3917-24
13. Wester HJ, U Keller M Schottelius, A Beer K Philipp-Abbrederis, F Hoffmann et al. Disclosing the CXCR4 expression in lymphoproliferative diseases by targeted molecular imaging. Theranostics. 2015;5:618-30
14. Jazin EE, S Soderstrom T Ebendal, D Larhammar. Embryonic expression of the mRNA for the rat homologue of the fusin/CXCR-4 HIV-1 co-receptor. J Neuroimmunol. 1997;79:148-54
15. Zou YR, AH Kottmann M Kuroda, I Taniuchi DR Littman. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595-9
16. https://clinicaltrials.gov/ct2/show/NCT01120457. 2010.
17. Herrmann K, M Schottelius C Lapa, T Osl A Poschenrieder, H Hanscheid et al. First-in-Human Experience of CXCR4-Directed Endoradiotherapy with 177Lu- and 90Y-Labeled Pentixather in Advanced-Stage Multiple Myeloma with Extensive Intra- and Extramedullary Disease. J Nucl Med. 2016;57:248-51
Author contact
![]() Corresponding author: Dr. Alexander Haug, Division of Nuclear Medicine, Dept. of Biomedical Imaging and Image-guided Therapy, Medical University of Vienna, 1090 Vienna, Austria. Tel.: +4314040039360; Fax: +4314040055320 Email: alexander.haugac.at
Corresponding author: Dr. Alexander Haug, Division of Nuclear Medicine, Dept. of Biomedical Imaging and Image-guided Therapy, Medical University of Vienna, 1090 Vienna, Austria. Tel.: +4314040039360; Fax: +4314040055320 Email: alexander.haugac.at
 Global reach, higher impact
Global reach, higher impact