13.3
Impact Factor
Theranostics 2019; 9(12):3398-3409. doi:10.7150/thno.33143 This issue Cite
Research Paper
Versatile and Finely Tuned Albumin Nanoplatform based on Click Chemistry
1. Department of Nuclear Medicine, Seoul National University Hospital, College of Medicine, Seoul, South Korea.
2. Graduate School of Convergence Science and Technology, Seoul National University, Seoul, South Korea.
3. Radiation Medicine Research Institute, Seoul National University College of Medicine, Seoul, South Korea.
4. Cancer Research Institute, Seoul National University, Seoul, South Korea.
5. Department of Biomedical Sciences, Seoul National University, Seoul, South Korea.
6. Department of RI Technology-Convergence, Korean Institute of Radiological & Medical Sciences (KIRAMS), Seoul, South Korea.
7. Isotope Production Division, Pakistan Institute of Nuclear Science & Technology (PINSTECH), P. O, Nilore, Islamabad.
8. Biomedical Research Institute, Seoul National University Hospital, Seoul, South Korea.
9. Department of Microbiology and Immunology, Institute of Endemic Disease, College of Medicine, Seoul National University, Seoul, South Korea.
10. Division of Pharmaceuticals and Clinical Development, DawonMedax Co., Ltd., Seoul, South Korea.
* These authors contributed equally.
Abstract
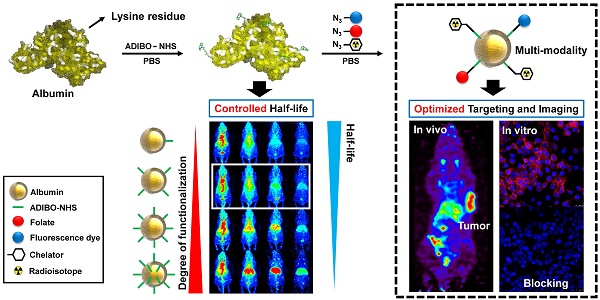
Albumin is one of the most attractive nanoplatforms for targeted imaging and drug delivery due to its biocompatibility and long circulation half-life. However, previously reported albumin-based nanoplatforms have shown inconsistent blood circulation half-life according to the modified methods, and the affecting factors were not well evaluated, which could hamper the clinical translation of albumin-based nanoplatforms. Herein, we developed a finely tuned click-chemistry based albumin nanoplatform (CAN) with a longer circulation half-life and an efficient tumor targeting ability.
Methods: CAN was synthesized in two steps. First, albumin was conjugated with ADIBO-NHS (albumin-ADIBO) by reacting albumin with various molar ratios of ADIBO. The number of attached ADIBO moieties was determined using matrix-assisted laser desorption ionization time of flight (MALDI-TOF). Second, the desired modalities including azide-functionalized chelator, a fluorescence dye, and folate were incorporated into albumin-ADIBO using strain-promoted alkyne-azide cycloaddition reaction (SPAAC reaction). The biodistribution and targeting efficiency of functionalized CANs were demonstrated in mice.
Results: The degree of functionalization (DOF) and resulting in vivo biodistribution was controlled precisely using the click chemistry approach. Specifically, the numbers of attached azadibenzocyclooctyne (ADIBO) moieties on albumin, the DOF, were optimized by reacting albumin with varying molar ratios of ADIBO with a high reproducibility. Furthermore, we developed a simple and efficient method to estimate the DOF using UV-visible spectrophotometry (UV-vis), which was further validated by matrix-assisted laser desorption ionization time of flight (MALDI-TOF). The biodistribution of CAN could be controlled by DOF, and CAN with an optimized DOF showed a long circulation half-life (> 18 h). CAN was further functionalized using a simple click chemistry reaction with an azide functionalized chelator, a fluorescence dye, and folate. 64Cu- and folate-labeled CAN (64Cu-CAN-FA) showed effective and specific folate receptor targeting in vivo, with an over two-fold higher uptake than the liver at 24 h post-injection.
Conclusions: Our development from the precisely controlled DOF demonstrates that an optimized CAN can be used as a multifunctional nanoplatform to obtain a longer half-life with radioisotopes and ligands, and provides an effective method for the development of albumin-based tumor theranostic agents.
Keywords: albumin, click chemistry, positron emission tomography, theranostics
 Global reach, higher impact
Global reach, higher impact