13.3
Impact Factor
Theranostics 2019; 9(11):3213-3222. doi:10.7150/thno.31854 This issue Cite
Review
Supramolecular Assemblies of Peptides or Nucleopeptides for Gene Delivery
Department of Chemistry, Brandeis University, 415 South Street, Waltham, MA 02453, USA.
Received 2018-11-28; Accepted 2019-3-26; Published 2019-5-18
Abstract
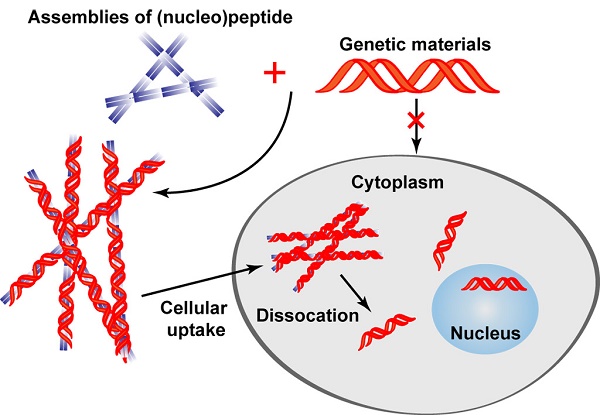
Using non-covalent interactions between nucleic acids (DNA, siRNA, miRNA, and mRNA) with peptides or nucleopeptides is a promising strategy to construct supramolecular assemblies for gene delivery and therapy. Comparing to conventional strategies for gene delivery, the assemblies of peptides or nucleopeptides provide several unique advantages: i) reversible interactions between the assemblies and the nucleic acids; ii) minimal immunogenicity; iii) biocompatibility. This field has advanced considerably in recent years so that it is worth summarizing the recent progresses and future challenges. In this review, we introduce the development of assemblies of peptides or nucleopeptides for applications in gene delivery and related fields. After introducing the promises of gene therapy and the current strategies for the delivery, we discuss the unique advantage of using peptide assemblies for gene delivery. Then we describe several representative strategies for gene delivery by the assemblies of peptides or nucleopeptides. Finally, we discuss the key factors for designing such assemblies for gene delivery, and speculate future directions and challenges in the field, particularly the rational design and the spatiotemporally controlled release in live cells.
Keywords: Self-assembly, Nanostructures, Gene delivery, Peptide, Nucleopeptide, Enzyme, Non-viral vector
Introduction
Gene therapy is believed to be a promising strategy for treating various diseases that are refractory to current methods. In principle, it is possible to modulate any sequence in genome or in transcriptome including both non-coding and coding RNA (e.g., mRNA or splicing regulated by siRNA or splice-correcting oligonucleotides) for therapeutic purposes. Among various approaches for editing genomes, CRISPR-Cas (clustered regularly interspaced short palindromic repeat-CRISPR-associated) is receiving the most recent attentions. On the other hand, almost 20 years after the discovery of RNAi, FDA approved patisiran [1], the first drug to harness RNA interference (RNAi) to silence disease-associated gene expression in 2017. While these exciting developments opens up a new adventure for discovering more potential gene therapies, the delivery of gene into cells remains an obstacle for gene therapy. Despite the numerous investigations of using exogenous nucleic acids for modulating gene expression or silencing one specific gene to treat different diseases in the past 30 years [2], the success in clinical trials is still limited due to the intrinsic limitations of nucleic acids and technical barriers. Nucleic acids contain negative charges, which disfavour cellular uptake. In addition, nucleic acids can be degraded easily by nucleases. Thus, efficient intracellular delivery of genes still is an unresolved issue in gene therapy.
To deliver gene safely and efficiently, both virus and non-virus delivery systems have been explored for delivering DNA, small interfering RNA (siRNA), microRNA (miRNA) mimics, and messenger RNA (mRNA). As the natural delivery vector, viruses have been investigated as delivery systems intensively, but few of them have entered clinic because of their intrinsic drawbacks, including low cargo capacity, immunogenicity, and limitation in large scale production [3-7]. To address these limitations [8-16], especially the limitations in immunogenicity and large scale production, researchers are developing non-viral vectors (e.g., nanoparticles, polymers, lipids, dendrimers, polypeptides, proteins, porous particles, dynamic covalent entities, and virus mimetics) for potential gene delivery. Despite the encouraging success achieved by these non-viral vectors, several obstacles, such as the low transfection efficiency, low capacity, difficulty in passing through physically barriers (i.e., endosome escaping), and poor cell selectivity, limit their further clinical applications [17, 18]. Among the non-viral vector for gene therapy, peptide based vectors have attracted increasing attentions in recent years because of their potentials for addressing the above limitations, and more importantly, peptides on their own exhibit minimal immunogenic, especially short peptides [19, 20].
The most explored peptide based vectors are cell penetrating peptides (CPPs) [21], which have multiple positively charged amino acid residues for binding nucleic acids and can translocate various nucleic acids (short or large DNA/RNA) from cell membrane to cytoplasm or nucleus. For example, Zhang and co-workers described multi-functional cell penetrating nona-arginine based peptide to form nanocomplex with nucleic acids for delivering miRNA mimics and the real-time monitoring the function of the mimics in live cells [22, 23]. Despite the certain success of CPPs achieved in gene therapy, limitations, such as cytotoxicity, proteolytic instability, and poor cell selectivity, still prevent their clinical applications. Another promising strategy is to develop peptide assemblies for gene delivery. Compared with individual peptides, peptide assemblies have several unique features, such as multivalent binding, dynamic control of affinity with nucleic acids, responsiveness for cellular cues, and long circulation time. Although peptide assemblies have been applied in various fields such as tissue engineering, wound healing, controlling cell death, analyte detection, and immune modulation in last two decades [24-32], their applications in gene delivery are only emerging in recent years so that it is worthwhile to summarize the progresses and future challenges.
This review intends to highlight the representative works of assemblies of peptides or nucleopeptide for gene delivery. First, we introduce the assemblies formed by positively charged peptides which interact with nucleic acids through electrostatic interactions. Second, we describe virus-like nanoparticles formed by peptides for gene delivery. Third, we highlight the enzyme-instructed assemblies of peptides for condensing DNA. Fourth, we discuss the strategy of introducing nucleobases into peptide assemblies for enhancing its affinity with nucleic acids for intracellular gene delivery and related applications. Finally, we give our consideration for rational design of peptide assemblies and the spatiotemporal controlled release of nucleic acids in live cells, as well as our perspectives about the challenges.
Assemblies of positively charged peptides interact with nucleic acids
Nucleic acids are negatively charged biopolymers, which inherently repel with anionic cell membrane component and thus exhibit limited ability to pass through cell membrane. Using cationic materials to interact with nucleic acids is a common strategy for reducing the charges of nucleic acids to deliver them into cytoplasm. The cationic materials not only improve the stability of nucleic acids, but also translocate them across cell membrane.
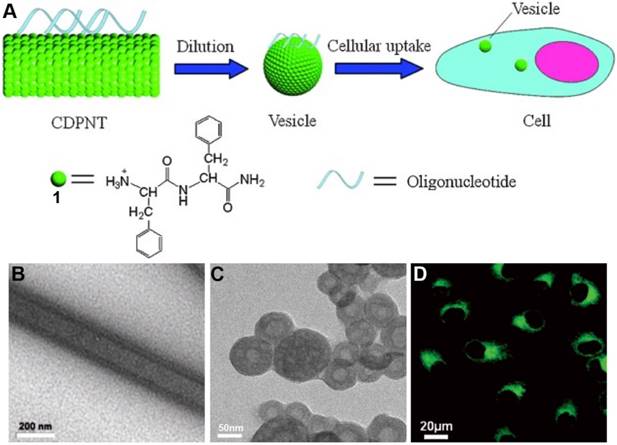
Li et al. [33] reported that a cationic dipeptide (H-Phe-Phe-NH2·HCl, 1, Figure 1A) can self-assemble into nanotube (CDPNT) in aqueous solution, which undergoes molecule rearrangement to form vesicles at physiological pH condition upon dilution. Being co-incubated with cells, the positively charged CDPNT can traverse cell membrane and enter the cells, which enable the delivery of exogenous oligonucleotides into cytoplasm. After demonstrating the formation of the nanotubes in aqueous solution at a high concentration (Figure 1B), the authors reported the use of TEM and fluorescent microscopy (in original paper) to verify its ability for binding ssDNA in tubular morphology. Negative staining indicated that the morphology transition from nanotubes to vesicles is concentration-dependent (Figure 1C). Cell experiment indicates that the mixture of CDPNT/ssDNA entered the cells and accumulated in cytoplasm (Figure 1D). Moreover, zwitterionic dipeptide (L-Phe-L-Phe) is unable to deliver ssDNA to cytoplasm, indicating the importance of electrostatic interaction and morphology transition for ssDNA delivery. Although the ssDNA is likely to be degraded inside cells, this work provides a simple model of self-assembly of cationic dipeptide for gene delivery, and could be applied for delivering other cargos (i.e. chemotherapy drugs).
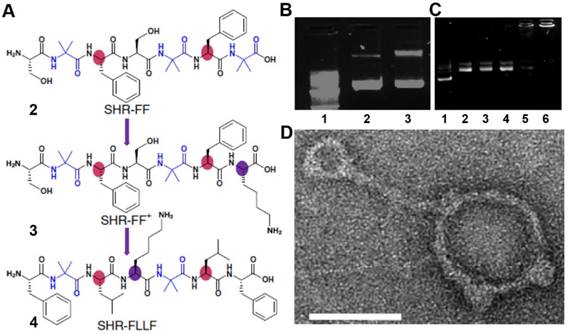
Although self-assembly of short peptides easily form ordered structures with various morphologies (e.g., nanofibers, nanotubes, and nanoparticles) [34-36], the interactions of the assemblies with biomacromolecules [37, 38] usually are inadequate because the secondary structures of the self-assembling peptides are limited mainly to β-sheet conformation, which lacks the precise orientation of amino acid residues needed for directed intermolecular organization. Inspired by that DNA binding proteins carries super-helical structures to recognize DNA for functions [39, 40], Gazit et al. [41] disclosed a rationally designed super-helical structure formed via the self-assembly of a heptad peptide and its application in DNA delivery. Based on the principle for designing canonical helical segment (known as a heptad repeat and designated as abcdefg), the authors introduced non-coding amino acid aminoisobutyric acid (Aib), a common strategy for increasing α-helical propensity of a peptide, into seven amino acid sequences (Figure 2A). To increase the intermolecular interactions, the authors put phenylalanine at the position of a and d for aromatic stacking. Their results indicated that 2 formed a preferential dimeric super-helical structure in aqueous solution, which was similar with leucine-zipper proteins. The authors further modified 2 by the inclusion of positively charged lysine at C-terminus to afford 3, which facilitated electrostatic interactions with the negatively charged DNA. Moreover, the authors also designed 4, which provided additional hydrophobic forces and imposes π-stacking between the peptides and nucleobases. Gel electrophoresis (Figure 2B and C) of the mixture (3 or 4 with DNA plasmid) revealed that 4 was the optimized candidate for inducing condensation of DNA plasmids, and TEM image verified the morphology (Figure 2D). If the DNA could be released easily from the complex, this study may lead to a new direction for designing super-helical nanostructures by the peptides that integrate helicity and non-covalent (including electrostatic) interactions for gene delivery.
(A) Proposed transition of the cationic dipeptide nanotubes (CDPNTs) into vesicles for oligonucleotide delivery. TEM image of the CDPNTs at the concentrations of (B) 10 mg/mL and (C) 1 mg/mL. (D) Fluorescence images of HeLa cells after incubation for 24 h with the complexes of CDPNTs and fluorescently labelled ssDNA (green). Adapted with permission from ref. 33. Copyright 2007, Wiley-VCH.
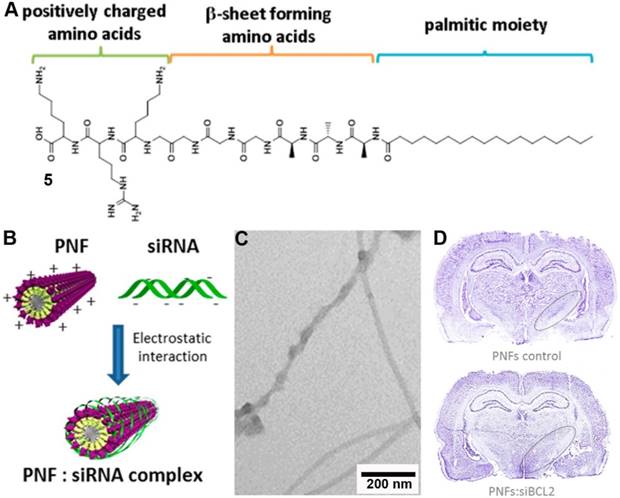
Engineering existing peptide nanostructures with positively charged amino acid residues for binding nucleic acids through electrostatic interaction, indeed, is a promising strategy for gene delivery. Typically, peptide nanofibers (PNFs) are the assemblies of short amphiphilic peptides that consist of a hydrophobic tail, a β-sheet forming amino acid sequence, and a hydrophilic group. Although PNFs typically found applications in tissue engineering and regenerative medicine [42-44], Kostarelos et al. recently explored them for gene delivery [45]. As shown by figure 3A, 5 is a surfactant-like peptide that self-assembles to form nanofibers in aqueous solution. The three positively charged amino acid residues (KRK) enable the assemblies to interact with the negatively charged siRNA electrostatically (Figure 3B). TEM image (Figure 3C) indicated nanofibers condensed siRNA around the surface of the nanofibers as evidenced by the dark objects presented on the surface of nanofibers. After verifying the biological activity of the complex of nanofibers and siRNA in vitro (gene knockdown in cell level), the authors further evaluated the in vivo efficiency of this vector by stereotactic injection in normal rat brains, which resulted in target gene silencing in specific loci of the brain (Figure 3D). Direct injection of vector complex and siRNA into brain could cause severe side effect (e.g., encephalitis) and the poor stability of this vector could also prevent its way to clinic. Using evolutionary assemblies of peptides with targeted motif or employing D-peptides may help address the above technique obstacles.
(A) Modification of the SHR-FF sequence to afford the positively charged single heptad repeat module containing single lysine residue. Colour regions display the significant variation in amino-acid residues. (B) Agarose-gel electrophoretic assay of DNA condensation by 3. Lane 1: 1 kb marker; lane 2: free DNA; lane 3: N/P 100. (C) Agarose-gel electrophoretic assay of DNA condensation by SHR-FLLF. Lane 1: 1 kb marker; lane 2: free DNA; lane 3: N/P 1; lane 4: N/P 20; lane 5: N/P 50: lane 6; N/P 100. (D) Magnified TEM image showed the presence of rod-like and toroidal structures of a peptide-DNA complex. Scale bar, 50 nm. Adapted with permission from ref. 41. Copyright 2015, Nature Publishing Group.
(A) Chemical structure of the peptide amphiphile palmitoyl-GGGAAAKRK. (B) Schematic representation of the PNFs:siRNA complex formation. (C) TEM images of PNF:siRNA complexes formed at 2.5:1 N/P ratio. (D) Light microscopy of Nissl-stained coronal sections at the level of the STN. Loss of STN neurons (circled areas) can be observed only in the PNF:siBCL2 treated brain. Adapted with permission from ref. 45. Copyright 2015, American Chemical Society
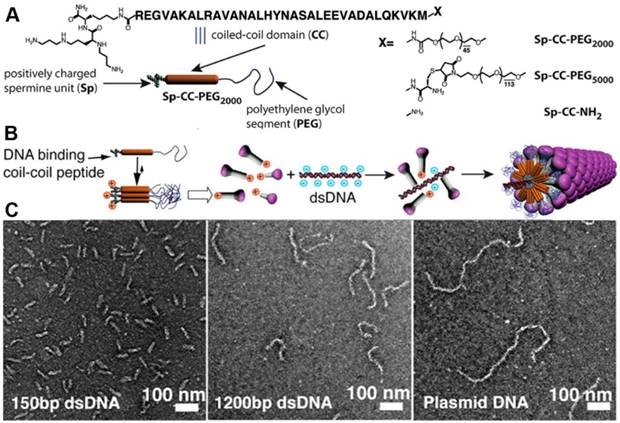
(A) Self-assembled mushroom shaped nanostructures created by the self-assembly of PEGylated coiled coil peptides modified by cationic spermine segments. (B) Schematic representation of the strategy to prepare synthetic filamentous viruses using a DNA template. (C) Effect of the size of the plasmid DNA template on the peptide/DNA complex length and morphologies. Adapted with permission from ref. 46. Copyright 2015, American Chemical Society
Virus-like peptide nanostructures for gene delivery
Viruses, consisting of capsid proteins and nucleic acids, are the naturally evolved vectors for gene delivery. Realizing the intrinsic weakness of viral vectors, such as immunogenicity, researchers have focused on the development of non-viral vectors, especially virus-like vectors. Among various virus-like vectors, assemblies of peptides have gained considerable interest in recent years due to its morphological similarity with virus. Virus capsid mimicry shares three basic components, a) a positively charged segment to interact with negatively charged nucleic acid; b) a self-assembly motif that is usually made of hydrophobic amino acid residues to stabilize the whole capsid through formation of secondary structures and intramolecular noncovalent interactions, and c) a hydrophilic unit to stabilize the whole system in environment (e.g., cell milieu). Although it is feasible to design peptide building blocks for binding nucleic acids based on the physical properties of nucleic acids, it is difficult to design a virus-like monodisperse structure with precisely controlled nanostructures. Stupp et al. described a novel strategy to create virus-like nanoparticles with defined length and morphology [46]. Figure 4A shows triblock molecules that consist of a nucleic acid binding ligand-spermine, a self-assemble coil-coil peptide, and long poly(ethylene glycol) chain. The authors discovered that the preformed coil-coil nanostructure was crucial for the formation of a virus-like monodisperse structure with nucleic acids (Figure 4B). Further experiments suggested that the length of PEG and the size of nucleic acids could influence the rigidity of the neutralized DNA template, resulting in different morphologies (Figure 4C). This work provides a new strategy to create precise nanostructures containing nucleic acids that have defined length. One potential drawback is that long PEG chains would likely decrease the transfection efficiency.
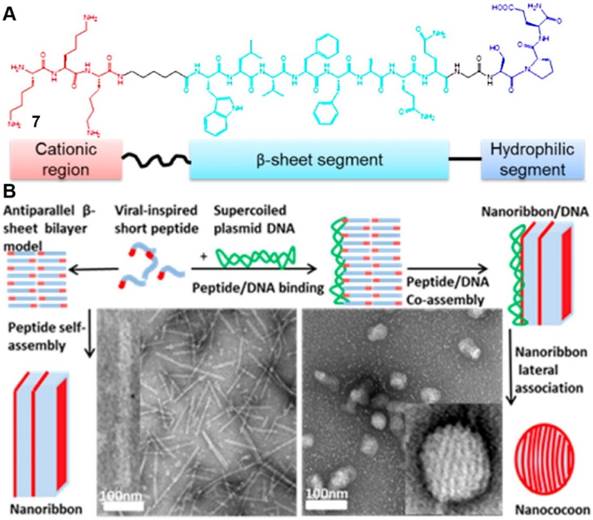
Simple and elaborate mimetic of virus capsid proteins by synthetic materials remains a challenge due to the sophisticated nanostructures and unique surface patterns of virus. Chau et al. employed well-developed β-sheet forming short peptides as a facile strategy for designing a simple system to mimic viral capsid proteins [47]. LVFFA, a central short hydrophobic β-sheet forming segment derived from amyloid β-peptide [48], serves as a short self-assembling motif [49, 50] that dominates the self-assembly of 7 (Figure 5A) to result in bilayer nanoribbons. The installation of positively charged amino acids (DNA binding domain) and hydrophilic amino acids at C-terminal (water preferred motif) provides other basic common structural motif for the capsid protein mimicking. Addition of 7 into the diluted solution of DNA results in nanococoons, which have a characteristic cocoon-like shape and a striped surface pattern (Figure 5B). Further mechanistic studies suggested that the co-assembly of 7 and DNA formed the nancocoons. In the presence of DNA, 7 self-assembles into nanoribbons which interact with DNA, and simultaneously wrap around DNA to afford stable nanostructures. The formed nanoribbons are extremely resistant to enzymes (i.e. DNase I, peptidases), indicating that the cleavage sites are inaccessible to both enzymes. Another work from the same group demonstrated that the formed nanoribbons achieved high gene-transfection efficacy in HEK293 cells [51]. Although the detailed mechanism of this observation remain to be elucidated, this work may lead to a facile approach to fabricate viral-mimetic nanostructure [52] with certain stability in vitro.
(A) Primary structure of K3C6SPD. The peptide consists of three modular segments as labeled. (B) Structural model and TEM images of the K3C6SPD and the mixture of K3C6SPD /DNA nanococoons. Adapted with permission from ref. 47. Copyright 2013, American Chemical Society.
Enzymatic instructed assemblies of peptide for condensing DNA
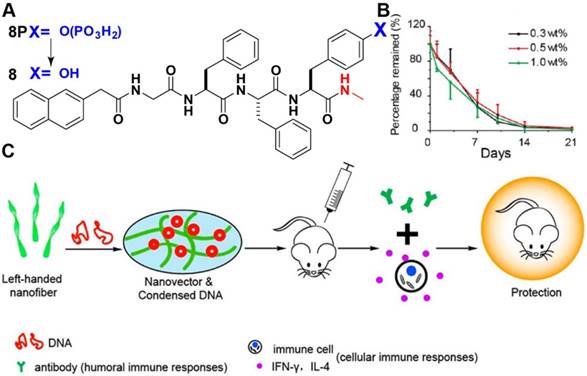
Since 2004 [53], the development of enzyme-instructed self-assembly (EISA) not only provides a biocompatibility strategy with small molecules to construct functional adaptive materials both in vitro and in vivo [54, 55], but also finds applications in various fields including drug delivery [56-59], wound healing [60], controlling cell fate [61-67], tissue engineering [68-70], active probes [71-75] and vaccine adjuvants [20, 76]. Different from above mentioned strategy to pre-organization of assemblies for gene delivery, using EISA to control peptide self-assembly to form elaborate nanostructures provides an alternative strategy for gene delivery in a safe and efficient way. EISA holds unique several advantages for gene delivery: 1) minimizing the use of positively charged amino acid residues; 2) reaction-diffusion controlled binding efficiency; 3) self-assembly enhanced cellular retention; 4) targeting specific cell type or organelles. Jiang and Yang et al. [77] reported that a peptide nanovector formed by EISA could condense DNA into co-assembled nanomaterials, resulting in strong immune responses against HIV. 8P is a substrate of alkali phosphatase (ALP), which becomes 8 upon hydrolysis by ALP (Figure 6A). During this process, the particle-like nanostructures turn into left-handed nanofibers, which co-assemble with DNA to condense DNA to nanoparticles efficiently. After demonstrating the biostability of nanofibers formed by 8P in vivo (Figure 6B), the authors discovered that the nanovectors strongly activated both humoral and cellular immune responses to a balanced level that rarely reported in the literatures and was crucial for HIV prevention and therapy. C-terminal engineering of 8P demonstrated the importance of methylamino group for efficient immune response in vivo. In their following effort [78], the authors found that in-situ formation of peptidic nanofibers without exogenous ALP also induced multiple crucial immunities against HIV DNA vaccine, suggesting in-situ formation of peptide nanofibers as a robust, safe, and efficient strategy for inducing crucial immune responses. This work illustrates the application of EISA and may lead the next-generation vaccines against different diseases.
(A) Chemical structures of the precursor and the corresponding hydrogelator used to form peptide based nanofibers for hydrogelation. (B) Radioactivity remaining (% of activity at day 0) at the injection sites of hydrogel containing 0.3, 0.5, and 1 wt% precursor at different time points. (C) Process of peptide-based nanofibrous hydrogels for enhancing immune responses of HIV DNA vaccines. Adapted with permission from ref. 77. Copyright 2014, American Chemical Society.
Assemblies of nucleopeptides for delivering DNA
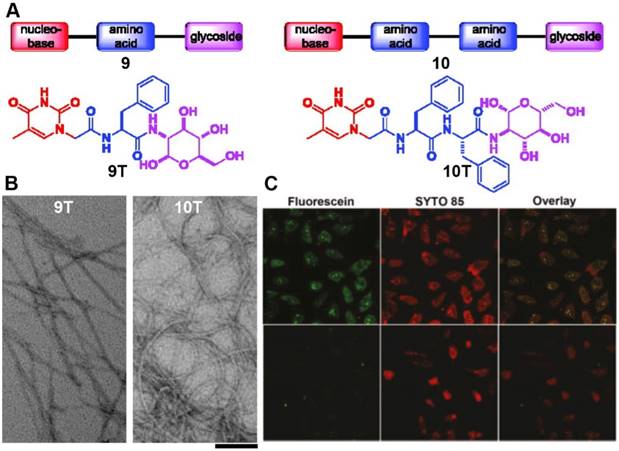
Besides peptides, another type of building blocks for constructing ordered nanostructures with protease resistant property is nucleopeptides. Generating from conjugates of nucleobases and peptides through covalent bonds, nucleopeptides exhibit good biostability, excellent biocompatibility, multiple functionalities, and readily self-assemble in aqueous solution. They have already been applied in biological and biomedical applications. Most importantly, bearing nucleobase(s), nucleopeptides are expected to interact with nucleic acids under physiological condition. As shown in a recent work [79], simply integrating nucleobase, amino acid, and glycoside in one molecule via covalent bonds (Figure 7A) results in assemblies of nucleopeptides. TEM images indicates that 9T and 10T form a characteristic morphology of the nanofibers (Figure 7B), which show good biocompatibility to mammalian cells and resist to proteases. Cell experiments (Figure 7C) indicate that these molecules interact with nucleic acids and facilitate the entry of nucleic acids into cytosol and nuclei of cells. This work may lead to a facile strategy to design safe and efficient vectors for delivering genes and other biomacromolecules.
Conclusion and outlook
Inspired from nature, non-viral gene delivery systems that mimic sophisticated structures of viral capsids now provide important scientific opportunities for gene therapies. Although variety of works of non-viral gene delivery have focused on the electrostatic interaction of vectors with nucleic acids in last two decades, utilizing assemblies of the peptides with minimum numbers of amino acid residues for gene delivery has emerged only recently. Besides aforementioned advantages of peptides, the abundant resources of peptide motifs for self-assembly (see protein data bank (PDB) [80, 81]) and the easily tailored physicochemical properties of peptide assemblies provide the unique opportunity for the development of assemblies of peptides. In addition to the strategy to design the assemblies of positively charged peptides for gene delivery, the promise of EISA for condensing DNA and assemblies of nucleopeptides for specific binding with DNA for gene delivery may hold great potentials. Further developing these two complementary strategies will contribute to gene therapy for curing genetic diseases with minimal adverse effects in the near future.
(A) Structures of the hydrogelators only consist of nucleobase, amino acid, and glycoside. (B) Transmission electron micrographs of the negatively stained hydrogels of 9T, 10T. Scale bar = 100 nm. (C) Fluorescence and bright field microscopy images showing the subcellular distribution of A10, which is labeled with fluorescein dye (green). Cell nuclei were stained with SYTO 85 (orange). (Top) 500 μM 9T and 1 μM FAM-A10 (fluorescein-labeled single strand oligonucleotide) incubated with HeLa cells for 24 h (Bottom). 1 μM FAM-A10 incubated with HeLa cells for 24 h. Adapted with permission from ref. 79. Copyright 2011, American Chemical Society.
The basic criteria for designing assemblies of peptides for gene delivery should always consider reproducibility, stability, and scalability [17, 82]. Some guiding principles are useful to design assemblies of peptides (or nucleopeptides) for delivery of nucleic acids: i) Balancing of hydrophobic and hydrophilic segments in designed molecule. Up to now, most of the peptide sequences with the ability of self-assembly are discovered serendipitously or based on the fragments of protein structures; these studies not only generate a large library of small molecules with self-assembling ability, but also provide useful information on rational designing assemblies of small molecules. For example, using established self-assembly motif, one can easily incorporate charged (positive or negative), hydrophobic, or aromatic amino acid residues for tuning the binding efficiency between peptide assemblies and nucleic acids, which would increase the gene delivery efficiency both in vitro and in vivo. ii) Introducing other non-covalent interactions (e.g., hydrophobic interaction and π-π interaction) as a complement of electrostatic interaction into assemblies could increase the stability of nanostructure formed by vectors and nucleic acids. Such a choice could minimize uncontrolled competitive interaction between vectors and biomolecules (e.g., proteins) and increase the transfection efficiency. iii) Considering microenvironments of different cell types and choosing suitable biological triggers. Engineering of assemblies for gene delivery to perform function requires the biological knowledge about the cellular microenvironment where the function is activated. For example, with the knowledge of mechanism of pH gradients on different status of endocytic pathway, scientists can design smart delivery system that incorporating acid-labile bonds with pH responsive property [83], which would increase the efficiency of gene therapy by escaping from endosome. In addition to pH trigger, other specific triggers (e.g., enzymes, redox, ATP) that can be easily incorporated into assemblies of peptides (or nucleopeptide) are only at the beginning, which require a careful consideration of more rational design. iv) Considering stability of assemblies in vivo. Stability of assemblies of the peptides is always the key factor for their bioactivity. Several strategies such as using D-amino acids, β-peptides, staple peptides, glycopeptides, and nucleopeptides could be useful for designing the assemblies to deliver genes [84].
Despite the promise of the use of assemblies of peptides or nucleopeptides for gene delivery, there are still challenges to be resolved before they meet clinical needs: i) delivering nucleic acids into a primary cell efficiently or precisely in vivo is required. Most of the reported works use immortalized cell lines in serum free conditions, which may lead to an overestimation of the delivery efficiency and false biocompatibility of the vectors. To predict the outcome of in vivo experiments, it is necessary to choose accurate in vitro models, such as primary cell culture, three dimensional (3D) cell cultures (3D spheroids). ii) Understanding of cellular uptake mechanism and intracellular trafficking of assemblies of peptides (or nucleopeptides) are important for clinical applications. iii) Selectively targeting specific tissues or organelles is needed. Design of non-viral vectors should consider the ability of targeting specific cell types and penetrating deep tissues. Therapeutic DNA expression requires efficient delivery of genes into nucleus, which remains a significant challenge. iv) Guiding principles to deliver multiple genes or combination with other strategies has yet to be developed. Despite these issue, nonetheless, the potential of assemblies of peptides (or nucleopeptides) for gene delivery has grown considerable with the researches. Further accumulation of the structural, biochemical, and cellular information of the assemblies of peptides or nucleopeptides would lead to opportunities for successful gene therapy.
Acknowledgements
This work was partially supported by NIH (CA142746) and NSF (DMR-1420382). ZF thanks the Dean's fellowship and NIH (F99CA234746).
Competing Interests
The authors have declared that no competing interest exists.
References
1. Mullard A. FDA approves landmark RNAi drug. Nat Rev Drug Discov. 2018;17:613-613
2. Stein CA, Castanotto D. FDA-approved oligonucleotide therapies in 2017. Mol Ther. 2017;25:1069-75
3. Thomas CE, Ehrhardt A, Kay MA. Progress and problems with the use of viral vectors for gene therapy. Nat Rev Genet. 2003;4:346-58
4. Kay MA, Glorioso JC, Naldini L. Viral vectors for gene therapy: the art of turning infectious agents into vehicles of therapeutics. Nat Med. 2001;7:33-40
5. Mingozzi F, High KA. Therapeutic in vivo gene transfer for genetic disease using AAV: progress and challenges. Nat Rev Genet. 2011;12:341-55
6. Giacca M, Zacchigna S. Virus-mediated gene delivery for human gene therapy. J Control Release. 2012;161:377-88
7. Cavazzana M, Bushman FD, Miccio A, André-Schmutz I, Six E. Gene therapy targeting haematopoietic stem cells for inherited diseases: progress and challenges. Nat Rev Drug Discov. 2019:1
8. Tseng Y-C, Mozumdar S, Huang L. Lipid-based systemic delivery of siRNA. Adv Drug Deliv Rev. 2009;61:721-31
9. Xu X, Wu J, Liu Y. et al. Ultra-pH-responsive and tumor-penetrating nanoplatform for targeted siRNA delivery with robust anti-cancer efficacy. Angew Chem Int Ed Engl. 2016;55:7091-4
10. Lächelt U, Wagner E. Nucleic acid therapeutics using polyplexes: a journey of 50 years (and beyond). Chem Rev. 2015;115:11043-78
11. Park TG, Jeong JH, Kim SW. Current status of polymeric gene delivery systems. Adv Drug Deliv Rev. 2006;58:467-86
12. Santos JL, Oliveira H, Pandita D. et al. Functionalization of poly (amidoamine) dendrimers with hydrophobic chains for improved gene delivery in mesenchymal stem cells. J Control Release. 2010;144:55-64
13. Pack DW, Hoffman AS, Pun S, Stayton PS. Design and development of polymers for gene delivery. Nat Rev Drug Discov. 2005;4:581-93
14. Ulrich Sb. Growing prospects of dynamic covalent chemistry in delivery applications. Acc Chem Res. 2019;19:510-9
15. Lostalé-Seijo I, Louzao I, Juanes M, Montenegro J. Peptide/Cas9 nanostructures for ribonucleoprotein cell membrane transport and gene edition. Chem Sci. 2017;8:7923-31
16. Louzao I, García-Fandiño R, Montenegro J. Hydrazone-modulated peptides for efficient gene transfection. J Mater Chem B. 2017;5:4426-34
17. Lostalé-Seijo I, Montenegro J. Synthetic materials at the forefront of gene delivery. Nat Rev Chem. 2018;2:258-77
18. Yin H, Kanasty RL, Eltoukhy AA. et al. Non-viral vectors for gene-based therapy. Nat Rev Genet. 2014;15:541-55
19. Purcell AW, Zeng W, Mifsud NA. et al. Dissecting the role of peptides in the immune response: theory, practice and the application to vaccine design. J Pept Sci. 2003;9:255-81
20. Rudra JS, Tian YF, Jung JP, Collier JH. A self-assembling peptide acting as an immune adjuvant. Proc Natl Acad Sci U S A. 2010;107:622-7
21. Lehto T, Ezzat K, Wood MJ, Andaloussi SE. Peptides for nucleic acid delivery. Adv Drug Deliv Rev. 2016;106:172-82
22. Xiao X, Wang X, Wang Y. et al. Multi-functional peptide-microRNA nanocomplex for targeted microRNA delivery and function imaging. Chemistry. 2018;24:2277-85
23. Xiao X, Wang X, Gao H. et al. Cell-selective delivery of microRNA with a microRNA-peptide conjugate nanocomplex. Chem Asian J. 2018;13:3845-9
24. Silva GA, Czeisler C, Niece KL. et al. Selective differentiation of neural progenitor cells by high-epitope density nanofibers. Science. 2004;303:1352-5
25. Holmes TC, de Lacalle S, Su X. et al. Extensive neurite outgrowth and active synapse formation on self-assembling peptide scaffolds. Proc Natl Acad Sci U S A. 2000;97:6728-33
26. Hendricks MP, Sato K, Palmer LC, Stupp SI. Supramolecular assembly of peptide amphiphiles. Acc Chem Res. 2017;50:2440-8
27. Ulijn RV, Smith AM. Designing peptide based nanomaterials. Chem Soc Rev. 2008;37:664-75
28. Branco MC, Sigano DM, Schneider JP. Materials from peptide assembly: towards the treatment of cancer and transmittable disease. Curr Opin Chem Biol. 2011;15:427-34
29. Gazit E. Self-assembled peptide nanostructures: the design of molecular building blocks and their technological utilization. Chem Soc Rev. 2007;36:1263-9
30. Langer R, Tirrell DA. Designing materials for biology and medicine. Nature. 2004;428:487
31. Shigemitsu H, Hamachi I. Design strategies of stimuli-responsive supramolecular hydrogels relying on structural analyses and cell-mimicking approaches. Acc Chem Res. 2017;50:740-50
32. Cheetham AG, Chakroun RW, Ma W, Cui H. Self-assembling prodrugs. Chem Soc Rev. 2017;46:6638-63
33. Yan X, He Q, Wang K. et al. Transition of cationic dipeptide nanotubes into vesicles and oligonucleotide delivery. Angew Chem Int Ed Engl. 2007;46:2431-4
34. Fleming S, Ulijn RV. Design of nanostructures based on aromatic peptide amphiphiles. Chem Soc Rev. 2014;43:8150-77
35. Mart RJ, Osborne RD, Stevens MM, Ulijn RV. Peptide-based stimuli-responsive biomaterials. Soft Matter. 2006;2:822-35
36. Du X, Zhou J, Shi J, Xu B. Supramolecular hydrogelators and hydrogels: from soft matter to molecular biomaterials. Chem Rev. 2015;115:13165-307
37. Adler-Abramovich L, Aronov D, Beker P. et al. Self-assembled arrays of peptide nanotubes by vapour deposition. Nat Nanotechnol. 2009;4:849-54
38. Adler-Abramovich L, Gazit E. The physical properties of supramolecular peptide assemblies: from building block association to technological applications. Chem Soc Rev. 2014;43:6881-93
39. Sun L, Young LN, Zhang X. et al. Icosahedral bacteriophage ΦX174 forms a tail for DNA transport during infection. Nature. 2014;505:432-5
40. Ciani B, Bjelić S, Honnappa S. et al. Molecular basis of coiled-coil oligomerization-state specificity. Proc Natl Acad Sci U S A. 2010. 1985 0-5
41. Mondal S, Adler-Abramovich L, Lampel A. et al. Formation of functional super-helical assemblies by constrained single heptad repeat. Nat Commun. 2015;6:8615
42. Sato K, Hendricks MP, Palmer LC, Stupp SI. Peptide supramolecular materials for therapeutics. Chem Soc Rev. 2018;47:7539-51
43. Boekhoven J, Stupp SI. 25th anniversary article: supramolecular materials for regenerative medicine. Adv Mater. 2014;26:1642-59
44. Cui H, Webber MJ, Stupp SI. Self-assembly of peptide amphiphiles: From molecules to nanostructures to biomaterials. Biopolymers. 2010;94:1-18
45. Mazza M, Hadjidemetriou M, de Lazaro I, Bussy C, Kostarelos K. Peptide nanofiber complexes with siRNA for deep brain gene silencing by stereotactic neurosurgery. ACS Nano. 2015;9:1137-49
46. Ruff Y, Moyer T, Newcomb CJ, Demeler B, Stupp SI. Precision templating with DNA of a virus-like particle with peptide nanostructures. J Am Chem Soc. 2013;135:6211-9
47. Ni R, Chau Y. Structural mimics of viruses through peptide/DNA co-assembly. J Am Chem Soc. 2014;136:17902-5
48. Lührs T, Ritter C, Adrian M. et al. 3D structure of Alzheimer's amyloid-β (1-42) fibrils. Proc Natl Acad Sci U S A. 2005;102:17342-7
49. Dong J, Canfield JM, Mehta AK. et al. Engineering metal ion coordination to regulate amyloid fibril assembly and toxicity. Proc Natl Acad Sci U S A. 2007;104:13313-8
50. Burkoth TS, Benzinger TL, Urban V. et al. Structure of the β-amyloid (10-35) fibril. J Am Chem Soc. 2000;122:7883-9
51. Ni R, Chau Y. Tuning the inter-nanofibril interaction to regulate the morphology and function of peptide/DNA co-assembled viral mimics. Angew Chem Int Ed Engl. 2017;56:9356-60
52. Kong J, Wang Y, Zhang J. et al. Rationally designed peptidyl virus-like particles enable targeted delivery of genetic cargo. Angew Chem Int Ed Engl. 2018;130:14228-32
53. Yang Z, Gu H, Fu D. et al. Enzymatic formation of supramolecular hydrogels. Adv Mater. 2004;16:1440-4
54. Feng Z, Zhang T, Wang H, Xu B. Supramolecular catalysis and dynamic assemblies for medicine. Chem Soc Rev. 2017;46:6470-9
55. Yang Z, Liang G, Xu B. Enzymatic hydrogelation of small molecules. Acc Chem Res. 2008;41:315-26
56. Gao Y, Kuang Y, Guo Z-F. et al. Enzyme-instructed molecular self-assembly confers nanofibers and a supramolecular hydrogel of taxol derivative. J Am Chem Soc. 2009;131:13576-7
57. Zhao F, Ma ML, Xu B. Molecular hydrogels of therapeutic agents. Chem Soc Rev. 2009;38:883-91
58. Wang H, Yang Z. Molecular hydrogels of hydrophobic compounds: a novel self-delivery system for anti-cancer drugs. Soft Matter. 2012;8:2344-7
59. Cheetham AG, Zhang P, Lin Y-a, Lock LL, Cui H. Supramolecular nanostructures formed by anticancer drug assembly. J Am Chem Soc. 2013;135:2907-10
60. Gao J, Zheng W, Zhang J. et al. Enzyme-controllable delivery of nitric oxide from a molecular hydrogel. Chem Commun (Camb). 2013;49:9173-5
61. Yang Z, Xu K, Guo Z, Guo Z, Xu B. Intracellular enzymatic formation of nanofibers results in hydrogelation and regulated cell death. Adv Mater. 2007;19:3152-6
62. Kuang Y, Shi J, Li J. et al. Pericellular hydrogel/nanonets inhibit cancer cells. Angew Chem Int Ed Engl. 2014;53:8104-7
63. Pires RA, Abul-Haija YM, Costa DS. et al. Controlling cancer cell fate using localized biocatalytic self-assembly of an aromatic carbohydrate amphiphile. J Am Chem Soc. 2015;137:576-9
64. Tanaka A, Fukuoka Y, Morimoto Y. et al. Cancer cell death induced by the intracellular self-assembly of an enzyme-responsive supramolecular gelator. J Am Chem Soc. 2015;137:770-5
65. Feng Z, Wang H, Wang S. et al. Enzymatic assemblies disrupt the membrane and target endoplasmic reticulum for selective cancer cell death. J Am Chem Soc. 2018;140:9566-73
66. Feng Z, Wang H, Xu B. Instructed assembly of peptides for intracellular enzyme sequestration. J Am Chem Soc. 2018;140:16433-7
67. Zhou J, Xu B. Enzyme-instructed self-assembly: a multistep process for potential cancer therapy. Bioconjug Chem. 2015;26:987-99
68. Wang H, Yang Z. Short-peptide-based molecular hydrogels: novel gelation strategies and applications for tissue engineering and drug delivery. Nanoscale. 2012;4:5259-67
69. Yang Z, Liang G, Wang L, Xu B. Using a kinase/phosphatase switch to regulate a supramolecular hydrogel and forming the supramolecular hydrogel in vivo. J Am Chem Soc. 2006;128:3038-43
70. Wang H, Shi J, Feng Z. et al. An in situ dynamic continuum of supramolecular phosphoglycopeptides enables formation of 3D cell spheroids. Angew Chem Int Ed Engl. 2017;56:16297-301
71. Gao Y, Shi J, Yuan D, Xu B. Imaging enzyme-triggered self-assembly of small molecules inside live cells. Nat Commun. 2012;3:1033
72. Liang G, Ren H, Rao J. A biocompatible condensation reaction for controlled assembly of nanostructures in living cells. Nat Chem. 2010;2:54-60
73. Kiyonaka S, Sada K, Yoshimura I. et al. Semi-wet peptide/protein array using supramolecular hydrogel. Nat Mater. 2004;3:58-64
74. Wang H, Feng Z, Del Signore SJ, Rodal AA, Xu B. Active probes for imaging membrane dynamics of live cells with high spatial and temporal resolution over extended time scales and areas. J Am Chem Soc. 2018;140:3505-9
75. Lee S, Xie J, Chen X. Peptides and peptide hormones for molecular imaging and disease diagnosis. Chem Rev. 2010;110:3087-111
76. Wang H, Luo Z, Wang Y. et al. Enzyme-catalyzed formation of supramolecular hydrogels as promising vaccine adjuvants. Adv Funct Mater. 2016;26:1822-9
77. Tian Y, Wang H, Liu Y. et al. A peptide-based nanofibrous hydrogel as a promising DNA nanovector for optimizing the efficacy of HIV vaccine. Nano Lett. 2014;14:1439-45
78. Liu Y, Wang H, Li D. et al. In situ formation of peptidic nanofibers can fundamentally optimize the quality of immune responses against HIV vaccine. Nanoscale Horiz. 2016;1:135-43
79. Li X, Kuang Y, Shi J. et al. Multifunctional, biocompatible supramolecular hydrogelators consist only of nucleobase, amino acid, and glycoside. J Am Chem Soc. 2011;133:17513-8
80. Berman HM, Westbrook J, Feng Z. et al. The protein data bank, 1999-. International tables for crystallography volume f: crystallography of biological macromolecules: Springer. 2006:675-84
81. Rose PW, Prlić A, Altunkaya A. et al. The RCSB protein data bank: integrative view of protein, gene and 3D structural information. Nucleic Acids Res. 2016;45:D271-D81
82. Leroux JC. Drug delivery: too much complexity, not enough reproducibility? Angew Chem Int Ed Engl. 2017;56:15170-1
83. Miyata K, Nishiyama N, Kataoka K. Rational design of smart supramolecular assemblies for gene delivery: chemical challenges in the creation of artificial viruses. Chem Soc Rev. 2012;41:2562-74
84. Wang H, Feng Z, Xu B. Bioinspired assembly of small molecules in cell milieu. Chem Soc Rev. 2017;46:2421-36
Author contact
![]() Corresponding author: email: whmeagleedu; bxuedu
Corresponding author: email: whmeagleedu; bxuedu
 Global reach, higher impact
Global reach, higher impact