13.3
Impact Factor
Theranostics 2019; 9(11):3107-3121. doi:10.7150/thno.34947 This issue Cite
Research Paper
Supramolecular therapeutics to treat the side effects induced by a depolarizing neuromuscular blocking agent
1. State Key Laboratory of Quality Research in Chinese Medicine, and Institute of Chinese Medical Sciences, University of Macau, Taipa, Macau, China.
2. Department of Pharmaceutics, College of Pharmacy, Third Military Medical University, Chongqing 400038, China.
3. State Key Laboratory of Chemical Engineering, Center for Chemistry of High-Performance & Novel Materials, Department of Chemistry, Zhejiang University, Hangzhou 310027, P. R. China.
4. School of Materials Science and Engineering, Nanjing University of Science and Technology, Nanjing 210094, China.
Received 2019-3-16; Accepted 2019-3-27; Published 2019-5-18
Abstract
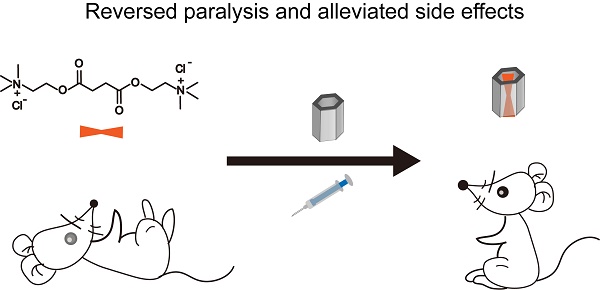
Succinylcholine (Sch) is the only depolarizing neuromuscular blocking agent widely used for rapid sequence induction in emergency rooms. Unfortunately, a variety of (sometimes lethal) adverse effects, such as hyperkalemia and cardiac arrest, are associated with its use, and currently there are no specific antidotes to reverse Sch or to treat these side-effects.
Methods: The binding behaviors of Sch and several synthetic receptors, including cucurbit[7]uril, sulfo-calix[4]arene and water-soluble carboxylatopillar[6]arene (WP[6]), were first investigated. With a mouse model, a leathal dose of Sch was selected for evaluation of the antidotal effects of these synthetic receptors on Sch induced mortality. The antidotal effects of a selected synthetic receptor, WP[6], on Sch induced cardiac arrhythmias, hyperkalemia, rhabdomyolysis and paralysis were subsequently evaluated with rat and mouse models. The reversal mechanism was also investigated at a cellular level.
Results: All of these macrocyclic molecules exhibited relatively high binding affinities with Sch in vitro. In a Sch-overdosed mouse model, immediate injection of these synthetic receptors right after Sch administration increased the overall survival rate, with WP[6] standing out with the most effective antidotal effects. In addition, administration of WP[6] also reversed the paralysis induced by Sch in a mouse model. Moreover, infusion of WP[6] to Sch-overdosed rats reduced the incidence of cardiac arrhythmia, inhibited the otherwise abnormally high serum potassium levels, and relieved the muscular damage. At the cellular level, WP[6] reversed the Sch induced depolarization and reduced the efflux of intracellular potassium.
Conclusion: Synthetic receptors, particularly WP[6], exhibited high binding affinities towards Sch, and presented a significant potential as supramolecular therapeutics to treat the various side effects of Sch by specifically sequestering Sch in vivo.
Keywords: succinylcholine, neuromuscular blocking agent, host-guest chemistry, pillararene, antidote
Introduction
Succinylcholine (Sch) is the only depolarizing neuromuscular blocking agent (NMBA) widely used in millions of patients annually in the current anesthesia practice. Attributed to its rapid onset (30 ~ 60 seconds) and brief duration of action (~ 10 minutes) [1], Sch has been used as the “gold standard” for rapid sequence induction (RSI) in emergency situations [2] and the management of laryngospasm [3]. Sch is also used to ensure patients' comfort and safety in electroconvulsive therapy [4, 5]. As a depolarizing NMBA, Sch is more resistant than acetylcholine (Ach) to degradation by cholinesterase at the neuromuscular junction and other Ach receptors (AchRs) sites, which may continuously depolarize the cells and induce repetitive excitation of the Ach receptors, thereby inducing the efflux of intracellular contents into the blood and causing a variety of side effects. These side effects may include prolonged apnea [6], myalgia [7], fasciculation induced gastric pressure increase [8], intraocular pressure increase [9, 10], hyperkalemia [11], cardiac arrhythmias [12-14], and malignant hyperthermia [15], some of which are life threatening, thus overdose or misuse of Sch may lead to death [16-19].
In the current clinical practice, treatment of the side effects induced by Sch mainly relies on the experience for management of similar symptoms and medical conditions observed in other diseases. For instance, according to the experience in management of hyperkalemia, calcium salt is administered to antagonize potassium effects on cardiac conduction caused by Sch induced hyperkalemia [20]. In addition, insulin in combination with dextrose (dextrose/insulin), or catecholamines or sodium bicarbonate is used to further promote the cellular uptake of potassium [21]. Similarly, dantrolene is administered to control Sch induced malignant hyperthermia [22-24]. However, the above-mentioned measures only focus on the symptoms, and patients need to be concurrently monitored with continuous electrocardiography during the treatment [20]. In addition, management of those symptoms often require several repetitive doses of these therapeutic drugs in combination, which may further induce other side effects, such as hypoglycemia (induced by dextrose/insulin), hypertension (induced by sodium bicarbonate) and muscle weakness (induced by dantrolene) [21, 22]. There has been no single therapeutic agent to deal with all Sch-induced side effects at the same time. Therefore, there is a significant medical need to develop a specific antidote to scavenge Sch, reverse its depolarization effects, and significantly alleviate its severe side effects simultaneously.
During the past decades, numerous supramolecular host molecules have been developed as artificial receptors, and their host-guest interactions as well as their derived supramolecular materials have shown significant potentials in biomedical applications [25-31]. Based on host-guest interactions, supramolecular host molecules may influence the physiochemical and biological properties of guest species, such as solubility, stability, toxicity and therapeutic efficacy [32-35]. As one of the most prominent example in this area, Sugammadex, a γ-cyclodextrin derivative, has been approved by more than 50 countries, including the European countries and the U.S.A., as a specific reversal agent for some of the non-depolarizing steroidal NMBAs including rocuronium and vecuronium [36, 37]. Based on its strong host-guest binding affinities with rocuronium or vecuronium, Sugammadex may selectively sequester these NMBAs in the circulatory system, and decrease their concentrations at the neuromuscular junction, thereby reversing neuromuscular blockage. Due to the potential allergic reactions of Sugammadex and its limitations to reverse mostly rocuronium and vecuronium but not the other NMBAs, Isaacs and co-workers recently developed C-shaped cucurbit[n]uril-type molecular containers, Calabadions, to selectively sequester and reverse neuromuscular blockage induced by not only steroidal NMBAs, but also benzylisoquinolines, in rats [38-41]. In spite of these great successes, neither Sugammadex nor Calabadions may effectively sequester and reverse Sch in vivo [40]. As Sch is still a clinically important, irreplaceable NMBA extensively used in anesthesiology, particularly during emergency surgeries for RSI, a specific antidote to counteract the potential side effects of Sch has been highly sought after.
The essential properties of supramolecular therapeutics as the reversal agent for Sch might include: (i) higher binding affinity with Sch than that of cholinergic receptors with Sch; (ii) higher binding affinity with Sch than that with other potential competitive molecules such as choline (Ch) and Ach (Figure 1A); (iii) good biocompatibility in vivo. In fact, it has been previously reported that a couple of synthetic receptors, cucurbit[7]uril (CB[7]) and sulfo-calix[4]arene (SC[4]A) (Figure 1A), could bind Sch with the binding constants in the order of ~106 and ~104 M-1, respectively, in aqueous solutions [42, 43]. And their good biocompatibility has recently been demonstrated in mammal models [44, 45]. However, an emerging synthetic receptor, water-soluble carboxylatopillar[6]arene (WP[6], Figure 1A), known to bind various aromatic and aliphatic cations [46-51], has never been reported for its complexation with Sch. In the present study, we investigated and discovered that WP[6] may bind Sch with the binding constant of ~106 M-1 in an aqueous solution, and it exhibited excellent biocompatibility in our systemic studies in a mouse model. Thus, we hypothesized that one or more of these synthetic receptors, CB[7], SC[4]A and WP[6], might fulfill the above-outlined requirements and serve as potential reversal agents of Sch for the treatment of Sch induced side effects, and more importantly may address the important medical needs that has been faced by our healthcare community for many decades.
Methods
Preparation of supramolecular compounds
Water-soluble carboxylatopillar[6]arene sodium salt (WP[6], C66H48O36Na12, molecular weight:1692.94 g/mol) was synthesized as described previously [46] and cucurbit[7]uril (CB[7]) (C42H42N28O14, molecular weight:1162.96 g/mol) was synthesized with the procedure reported by references [52-54]. Sulfo-calix[4]arene sodium salt (SC[4]A) (C28H20O16S4Na4, molecular weight:832.67 g/mol) was provided by Dr. Dongsheng Guo (Nankai University, China).
Modeling studies of WP[6] and Sch binding
The binding conformations between host molecules and Sch were simulated with AutoDock Vina [55]. The center of the search space of WP[6] and Sch was set to 8.337 Å, -10.342 Å and -10.975 Å (x, y, z). A grid map of dimensions 40 Å × 40 Å × 40 Å with a grid space of 0.375 Å was set. One hundred GA (genetic algorithm) runs was set, and all other parameters were the default values by AutoDock Vina. Conformational searching was performed by the Lamarckian genetic algorithm (LGA). The structure of the complex with lowest energy was re-optimized with ChemBio3D Ultra 14.0.
Binding behaviors of synthetic receptors and succinylcholine (Sch), acetylcholine (Ach) and choline (Ch)
The binding affinities and thermodynamic parameters of host molecules and Sch (Succinylcholine chloride dihydrate, >98%, aladdin®), Ach (Acetylcholine chloride, 99%, aladdin®) and Ch (Choline chloride, 99%, aladdin®) were determined by Isothermal Titration Calorimetry (ITC, Malvern MicroCal PEAQ, Malvern, Worcestershire, UK). To determine the binding affinity of WP[6] and Sch in aqueous solution, 200 µL of aqueous WP[6] solution (0.15 mM) was placed into the sample cell, and a 2 mM aqueous Sch solution was added in a series of 19 injections (2 μL each) as the heat evolved was recorded at 25.0 °C. The time intervals for each titration was set at 150 s. Thermodynamic parameters analysis was conducted with the “one set of binding sites” mathematic model that built in the software. Similar methods were utilized to determine the binding affinities of host molecules and Sch, Ach and Ch in aqueous solution or pH 7.4 phosphate-buffered saline (PBS) solution containing 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, and 1.8 mM KH2PO4. 1H-NMR was also conducted to determine the binding behavior of WP[6] and Sch in water.
Biocompatibility evaluation of WP[6] in a mouse model
Female Balb/c mice (8-10 weeks old) with body weights of 20-25 g were randomly separated into groups (n = 6 for each group). In the i.v. WP[6] group, 60 mg/mL WP[6]/saline solutions were prepared and passed through a 0.22 µm filter membrane and mice were intravenously administered with 50 µL solution/10 g body weight. Mice were intravenously administered with 50 µL saline/10 g body weight in the control group. Mice were weighed every two days, and their behaviors were observed for any signs of illness each day. After 14 days, blood samples were collected for the hematological analysis. In addition, serum was separated for the hepatic and renal functional markers test. Major organs including the heart, liver, kidneys, lungs and spleen were collected. Sections of major organs were prepared and stained with hematoxylin-eosin (H&E) for histopathological analysis.
Lethal Sch poisoning mouse model
Female Balb/c mice (8-10 weeks old) with body weights of 20-25 g were randomly separated into five groups (n = 6 for each group). Sch/saline solutions with concentration of 80, 100, 120, 150 and 200 µg/mL were prepared. Mice were intravenously injected with Sch (100 µL/20 g) at the dose of 0.4, 0.5, 0.6, 0.75 and 1 mg/kg and their behaviors were observed, and survival rates were recorded for 24 hours.
Evaluation of the antidotal effects of the host molecules in a lethal Sch poisoning mouse model
Female Balb/c mice (8-10 weeks old) with body weights of 20-25 g were randomly separated into several groups (n = 6 for each group). WP[6], SC[4]A and CB[7] solutions were prepared with saline. Mice were firstly i.v. injected with Sch at the dose of 0.75 mg/kg and i.v. administered with WP[6] (at the dose of 10, 20 and 50 mg/kg), SC[4]A (at the dose of 20 and 50 mg/kg) and CB[7] (100 mg/kg) immediately. Mice were i.v. injected with 100 µL saline per 20 g as the control group. Survival mice were recorded within 24 hours. To further explore the safety of mice in the group with 100% survival rate, body weights of mice were recorded, and the behavior of mice was observed daily for any sign of illness. After 14 days, mice were sacrificed, and blood was collected for the determination of the hematological parameters. Major organs were also collected for histopathological analysis.
Electrocardiogram (ECG) recording
Male Sprague Dawley rats (10-12 weeks old) with the body weight of 300-360 g were used for the ECG recording. Rats were anesthetized and after tracheotomy, respirations were controlled with a respirator (2 mL/100 g, 60 breaths/min). ECG was continuously monitored by positioning electrodes on all four limbs using a Mindray monitor (BeneHeart R3A). An indwelling needle was applied for the administration of drug within jugular vein. Rats were injected with saline or WP[6] at the dose of 20 or 50 mg/kg at 30s after Sch administration (0.9 mg/kg, 2 times of ED90 [40]).
Potassium levels in serum
Male Sprague Dawley rats (10-12 weeks old) with the body weight of 300-360 g were anesthetized, and after tracheotomy, respirations were controlled with a respirator (2 mL/100 g, 60 breaths/min). An indwelling needle was applied for the blood collection within the right jugular vein. Rats were injected (via the left jugular vein) with saline or WP[6] at the dose of 20 or 50 mg/kg at 30s after Sch administration (0.9 mg/kg). Blood was collected before or at 5, 10, 15 and 25 min after Sch administration and serum was separated by centrifuging at 2000 ×g for 15 min. And potassium levels in the serum was determined using a XD 687 ELECTROLYTE ANALYZER (Shanghai Xunda Medical Instrument Co., Ltd, China).
Evaluation of the rhabdomyolysis in a rat model
Male Sprague Dawley rats (10-12 weeks old) with the body weight of 300-360 g were anesthetized, and after tracheotomy, respirations were controlled with a respirator (2 mL/100 g, 60 breaths/min). Rats were injected with 1.0 mg/kg Sch (250 µL/kg) via the right anterior tibial muscle (i.m.) [56], and after 30 s, rats were then injected with saline (250 µL/kg) or WP[6] (20 mg/kg and 50 mg/kg) at the same site. The i.m. route was employed in this study in order to have a high concentration of Sch in the local muscle, leading to rhabdomyolysis, to facilitate the antidotal efficacy study of WP[6]. After 15 min, blood was collected, and serum was separated for determining the levels of urea, uric acid, creatinine (Crea), creatine kinase (Mindray BS 800) and myoglobin (fluorescence immunochromatography assay, GUANGZHOU WONDOFO BIOTECH CO., LTD) and the right anterior tibial muscle was collected for the histopathological analysis.
Mobility recovery recording in a mouse model
The movement behavior was conducted with the Rotarod rotor (IITC Life Science). The rotor parameter was set from 4 to 40 rpm in 180 s. Female Balb/c mice (8-10 weeks old) with 20-25 g weight were put on the Rotarod rotor and trained 5 times for 2 consequent days. And at day 3, mice were firstly trained for one time. And mice were i.v. administered with 20 mg/kg or 50 mg/kg WP[6] or saline at 30 s after 0.5 mg/kg Sch administration. Mice were then put on the rotor, and the time was recorded when the mice could move on the rotor for 30 s successfully.
Cell line
Rat skeletal muscle myoblast L6 cell line was purchased from COBIOER BIOSCIENCES CO. LTD (Nanjing, Jiangsu, China) and cultured in complete Dulbecco's modified eagle medium (DMEM, Gibco) supplemented with 10 % fetal bovine serum (FBS, Gibco) and 1 % penicillin-streptomycin (Sigma-Aldrich).
Determination of the plasma membrane potential
Flow cytometry
After removal from the culture flask, 2×105 L6 cells were incubated in HBSS (Hank's Balanced Salt Solution) containing 10, 100 and 1000 mM Sch for 10 min at 37 ℃, and after centrifuged, cells were then incubated with 200 nM Bis-(1,3-Dibutylbarbituric Acid)Trimethine Oxonol (DiBAC4(3), Sigma-Aldrich) for 20 min at 37 ℃, and the cell suspension were then maintained, and flow cytometry (BD Accuri C6) was used for determining the fluorescence of cells. To determine the influence of WP[6] on the plasma membrane potential, 2×105 L6 cells were co-incubated with 100 µM Sch and various concentration of WP[6] (0, 100, 200, 500 and 1000 µM) or 1000 µM WP[6] in HBSS for 10 min at 37 ℃. And the plasma membrane potential was then determined using the same method described above.
Confocal laser scanning microscopy (CLSM)
2×105 L6 cells were cultured in 12-well plates containing a small cover glass. After 12 h, the culture medium was removed, and cell were then co-incubated with 100 µM Sch and various concentration of WP[6] (0 and 1000 µM) in HBSS for 10 min at 37℃. After washed with HBSS, cells were then incubated with 200 nM DiBAC4(3) in HBSS for 20 min at 37℃. Cells were then fixed with 4% paraformaldehyde for 15 min and stained with 4',6-diamidino-2-phenylindole (DAPI) for 2 min. CLSM (ZEISS LSM 800) were then used for the fluorescence observation.
Determination of intracellular potassium levels
5×105 L6 cells with 2 mL medium were seeded in 6-well plates. After 12 h, the culture medium was removed, and cells were then co-incubated with 100 µM Sch and various concentration of WP[6] (0, 100, 200, 500 and 1000 µM) or 1000 µM WP[6] in HBSS for 10 min at 37 ℃. For the control group, cells were incubated with 2 mL HBSS for 10 min at 37 ℃. After washed with saline, cells were then lysed with 400 µL 1% TritoonX-100 in saline (containing 1% proteinase inhibitor cocktail, Sigma-Aldrich). The lysis was then centrifuged at 10000 ×g for 10 min and the supernatant was collected for determination of potassium levels (ELECTROLYTE ANALYZER) and total protein content (BCA assays, Beyotime Biotechnology). The ratio of potassium levels and total protein content was calculated, and the results were presented as the relative content of intracellular potassium of the control group.
Blood parameters analysis
An automated hematology analyzer (Sysmex KX-21, Sysmex Co., Japan) was used to determine whole blood cell (WBC), red blood cell (RBC), platelet (PLT) and hemoglobin (HGB). The serum concentrations of biomarkers including alanine aminotransferase (ALT), aspartate aminotransferase (AST), Crea, urea and uric acid (UA) were quantified with Roche Cobas C501 (Roche Co., Switzerland).
Histology
Tissues were firstly fixed in 4 wt% paraformaldehyde for 24 h, and then embedded in paraffin and cut into 6-μm sections. Sections were then stained with H&E and examined with an optical microscope.
Statistical analysis
Statistical analysis was done with PASW Statistics 18.0 software. One-way ANOVA was used for experiments consisting of more than two groups. Two-tailed, unpaired Student's t-tests were performed for the comparisons between two sample sets. Statistical significance was considered as P < 0.05.
Study approval
Male Sprague Dawley rats and female Balb/c mice were purchased from the Animal Center of the Third Military Medical University. The animal work was approved by the Animal Ethics Committee at Third Military Medical University (a.k.a. “Army Medical University”) and was conducted in line with the Animal Management Rules of the Ministry of Health of the People's Republic of China (No. 55, 2001) and the guidelines for the Care and Use of Laboratory Animals of the Third Military Medical University.
Results
Binding behaviors between the synthetic receptors and Sch
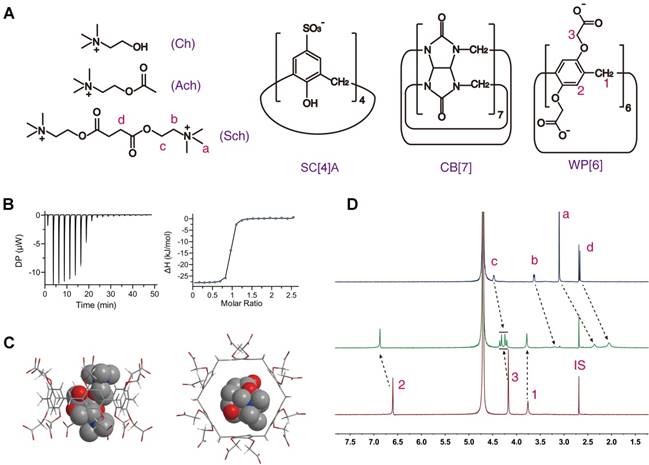
The binding behaviors of Sch with CB[7] and SC[4]A have been studied in previous works, with the binding constant being ~106 and ~104 M-1, respectively, in aqueous solutions [42, 43]. For studying the binding behavior between Sch and WP[6], we firstly determined their binding constant at 25 ˚C using isothermal titration calorimetry (ITC), to be ~3.42 × 106 M-1 in water (Figure 1B and Table 1). The binding geometry of Sch and WP[6] were simulated with AutoDock Vina [55]. As shown in Figure 1C, the entire Sch molecule is precisely located in the cavity of WP[6]. The complexation of WP[6] and Sch was also studied by 1H-NMR spectroscopy. As shown in Figure 1D, all Sch proton resonances shifted to upper field significantly when 1 molecular equiv. of WP[6] was mixed with Sch (1 mM) in D2O, suggesting the encapsulation of Sch inside the electron-rich cavity of WP[6], consistent with the optimized complex structure simulated by AutoDock Vina.
On the other hand, the binding constants between Sch and AchRs are at the level of ~103 M-1 (muscarinic acetylcholine receptors, mAchRs) [57] and ~104 M-1 (nicotine acetylcholine receptors, nAchRs) [58, 59] in pH 7.4 buffer solutions (Table S1). Therefore, the binding affinities between Sch and synthetic receptors should be higher than ~104 M-1 in physiological environments to scavenge Sch effectively from the AchRs, thereby reducing the Sch induced side effects and toxicities. We subsequently determined the binding affinities between Sch and each of the three synthetic receptors at 25 ˚C in PBS buffer solution (pH 7.4) using ITC, and found that the binding constants of Sch and WP[6], SC[4]A and CB[7] are 2.79×105, 7.52×104 and 1.00×105 M-1, respectively, in PBS (Figures S1-S3 and Table 1), all higher than the binding affinities between Sch and AchRs. These results suggested that WP[6], SC[4]A and CB[7] might scavenge Sch effectively from the AchRs in vivo.
Meanwhile, several Sch structurally relevant molecules, such as Ach (EC50 6.04~10.36 µM) [60] and Ch (10~15 µM) present around muscular nAchRs and in the blood [61], may also bind these synthetic receptors competitively. An effective Sch scavenger must have a high M value (Ka Sch/ Ka Ach and Ka Sch/ Ka Ch) to be able to selectively scavenge Sch in the presence of Ach and Ch. The binding affinities between each of these receptors and Ach and Ch, respectively, were subsequently determined at 25 ˚C in PBS buffers and water using ITC, and the M value calculated are all higher than 2.00 (Table 1), suggesting that WP[6], SC[4]A and CB[7] may preferentially bind Sch even in the presence of Ach and Ch around the AchRs sites or in the circulatory system.
Chemical structures and binding behaviors of WP[6] and Sch. (A) Chemical structures of WP[6], SC[4]A, CB[7], Ch, Ach and Sch. (B) ITC titration of WP[6] and Sch in water at 25 °C. One binding site model was utilized to fit the data, affording an association constant Ka of 3.42 × 106 M-1 (ΔH: -28.2 kJ/mol, ΔG: -37.3 kJ/mol and -T ΔS: -9.16 kJ/mol). (C) Energy-optimised inclusion complexes of WP[6] and Sch. (D) 1H-NMR spectra (400 MHz, D2O, room temperature).Top: Sch (0.50 mM); Middle: WP[6] (0.50 mM) and Sch (0.50 mM); Bottom: WP[6] (0.50 mM). IS: internal standard (tetramethylsilane).
Biocompatibility evaluation of WP[6] in a mouse model
To detoxify Sch induced side effects, the antidote, at an effective dose, must be safe and would not induce further adverse side effects. The good biocompatibility of both CB[7] and SC[4]A has recently been demonstrated and a single intravenous (i.v.) dose of 150 mg/kg CB[7] or 100 mg/kg SC[4]A would not induce toxicity in mice models [44, 45]. Herein, we performed a toxicological study in mice to evaluate the toxicity and biocompatibility of WP[6] in vivo. In our studies, severe spasm was induced immediately in mice that were i.v. administered with WP[6] at the dose of either 350 or 400 mg/kg, and the mice died shortly after injections. With the dose reduced down to 300 mg/kg, no immediate abnormal behaviors or medical conditions were observed, and we therefore evaluated the acute toxicity of WP[6] in mice with the i.v. dose of 300 mg/kg. All mice that received WP[6] at this dose level or saline were alive and weight changes of the mice were comparable between two groups during our 14-day follow-up (Figure S4A). On Day 14 after the administration of WP[6], the mice were sacrificed for collection of the blood and major organs including the heart, liver, spleen, lungs and kidneys. Hematological parameters including whole blood cell (WBC), red blood cell (RBC), platelet (PLT) and hemoglobin (HGB) as well as biomarkers for hepatic and renal functions including alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatinine (Crea) and urea, from WP[6]-treated group showed no significant differences with those from the control group administered with saline (Figure S4B-D). Moreover, the histopathological analysis of the major organs revealed no detectable injuries (Figure S5). These results suggest a good biocompatibility profile of WP[6] in mice at a relatively high i.v. dose of 300 mg/kg.
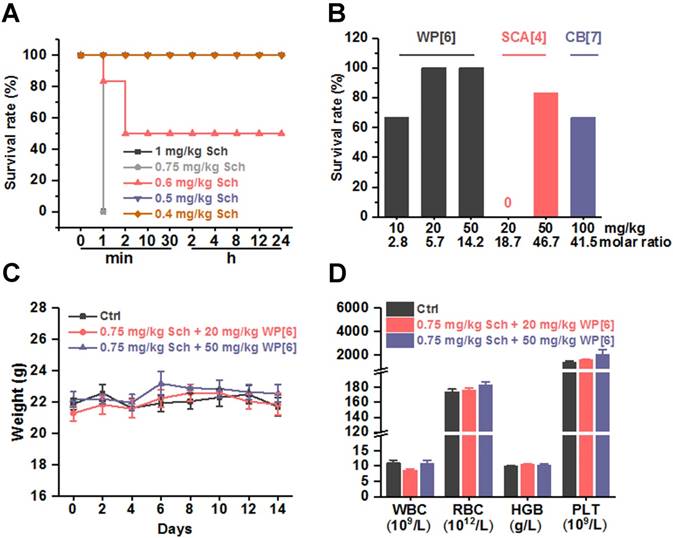
Improved survival in a lethal Sch-poisoning mouse model
After having determined the binding parameters between Sch, Ach, Ch and each of the synthetic receptors, and have concluded the good biocompatibility of these receptors in mice, we conducted experiments to evaluate whether these synthetic receptors could reverse Sch induced side effects and toxicity. The toxicity of Sch was first evaluated in mice in order to choose an appropriate dose of Sch in this preclinical study (Figure 2A). Systemic fasciculation and paralysis were induced immediately, when mice were i.v. administered with Sch at a dose of 0.4 or 0.5 mg/kg. Paralysis lasted for several minutes before the mice recovered back to normal mobility. When mice were i.v. administered with Sch at the dose of 0.6 mg/kg, severe systemic fasciculation was induced immediately, followed by respiratory depression, mydriasis and encopresis, and eventually resulted in a 50% mortality rate. When the dose was elevated to 0.75 and 1 mg/kg, all mice died in 1 min. With these results obtained, the lethal dose of Sch, 0.75 mg/kg, was chosen for evaluation of the antidotal effects of synthetic receptors on Sch induced mortality. Several groups of mice were i.v. injected respectively with WP[6], SC[4]A and CB[7] at various doses immediately after the administration of 0.75 mg/kg Sch. As shown in Figure 2B, 4 of 6 mice survived when mice were administered with 10 mg/kg WP[6] (molar ratio of WP[6]/Sch = 2.8:1), and all mice survived when the dose of WP[6] was either 20 or 50 mg/kg (WP[6]/Sch = 5.7:1 and 14.2:1, respectively), suggesting that WP[6] may fully reverse lethal dose Sch induced death when the given dose is higher than 5.7 equiv. of Sch. The antidotal effects were likely attributed to the specific complexation and sequestration of Sch by WP[6] in the blood and neuromuscular junctions, leading to dissociation of Sch from AChRs. Conversely, even 50 mg/kg SC[4]A (molar ratio of SC[4]A/Sch = 46.7:1) and 100 mg/kg CB[7] (molar ratio of CB[7]/Sch = 41.5:1) did not fully reverse the mortality induced by the lethal dose Sch. Therefore, WP[6] stood out from these synthetic receptors and might act effectively to reverse a variety of other Sch induced side effects. We subsequently evaluated the mice that fully survived after treatment with WP[6] post injection of a lethal dose Sch. The body-weights were comparable between the control group and treatment groups during the 14-day follow up (Figure 2C). These mice were sacrificed on Day 14, and the hematological parameters including WBC, RBC, HGB and PLT of the blood taken from the mice were also comparable between the treatment and control groups (Figure 2D). Moreover, the histopathological analysis of major organs of these mice showed no detectable injuries in both groups (Figure S6). Therefore, WP[6] was selected for further, systemic evaluations of therapeutic effects against Sch induced side effects and toxicities.
Synthetic receptors reversed Sch induced death in a mouse model. (A) Survival rates of mice that were i.v. administered with various doses of Sch. n = 6 for each group. (B) Survival rates of mice that were i.v. treated with various doses of synthetic receptors immediately after the i.v. administration of a lethal dose of Sch (0.75 mg/kg). n = 6 for each group. (C) Weight changes of mice that received the treatment of WP[6] immediately after i.v. administration of 0.75 mg/kg Sch. n = 6 for each group. (D) Hematological parameters of the blood from mice that were sacrificed at Day 14 after received the treatment of WP[6] immediately after the i.v. administration of a lethal dose of Sch (0.75 mg/kg). n = 6 for each group.
Binding parameters of Sch, Ach, Ch and synthetic receptors. (Ka, M-1)
| Sch (water) | Sch (PBS) | Ach (water) | Ach (PBS) | Ch (water) | Ch (PBS) | M1 (PBS) | M2 (PBS) | |
|---|---|---|---|---|---|---|---|---|
| WP[6] | 3.42×106 | 2.79×105 | 2.33×105 | 3.55×104 | 5.24×105 | 5.99×104 | 7.86 | 4.66 |
| SCA[4] | 7.30×105 | 7.52×104 | 1.64×104 | 9.35×103 | 3.33×105 | 1.99×104 | 8.04 | 3.77 |
| CB[7] | 1.38×106 | 1.00×105 | 5.21×104 | 1.41×104 | 9.80×105 | 4.31×104 | 7.09 | 2.32 |
M1: Ka Sch/ Ka Ach; M2: Ka Sch/ Ka Ch. Determined at 25 ˚C by ITC.
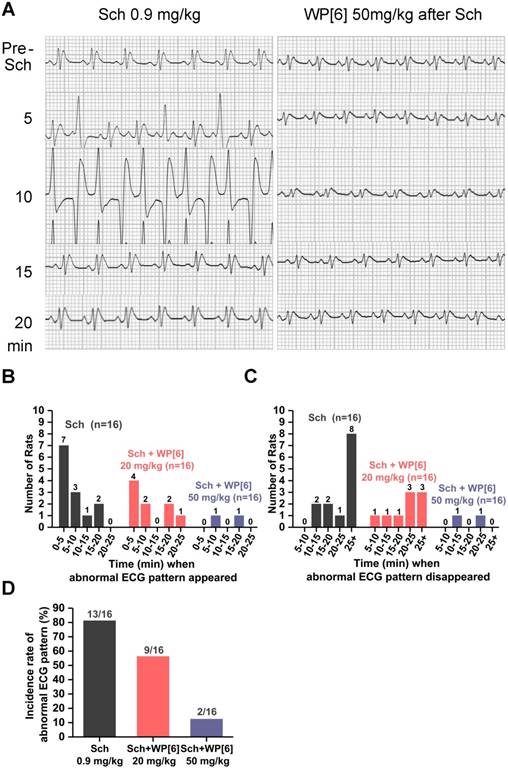
WP[6] reversed the Sch induced abnormal ECG pattern in a rat model. (A) Representative ECG patterns of rats that i.v. administered with or without 50 mg/kg WP[6] at 30 s after Sch administration. (B and C) Time when the abnormal ECG pattern appeared (B) and disappeared (C) after rats were i.v. administered with or without WP[6] (20 or 50 mg/kg) at 30 s after Sch administration. n = 16 for each group. (D) Incidence rate of the abnormal ECG pattern of rats that i.v. administered with or without WP[6] (20 or 50 mg/kg) at 30 s after Sch administration. n = 16 for each group.
Reduced incidence of Sch induced cardiac arrhythmias in a rat model
Electrocardiogram (ECG), a graph of the heart's electrical activity, is often recorded continuously during an operative procedure under anesthesia to monitor the potential side effects of NMBA. It is well documented that administration of Sch is associated with cardiac arrhythmias or cardiac arrest in both adults and children, sometimes leading to mortality [20, 62-64]. To test whether WP[6] could reverse Sch induced cardiac arrhythmias, we recorded ECG of rats before and after administration of Sch with or without the treatment of WP[6] shortly after Sch administration. Rats were i.v. administered with 0.9 mg/kg Sch (2 times of ED90 [40]), and abnormal ECG patterns, such as increased amplitude of QRS complex and premature ventricular contraction, occurred within 5 min after the administration, followed by bigeminy and ST-T waves abnormalities in the next 10 min (Figure 3A). The ECG pattern came to be normal at 20 min post administration and started to exhibit wave trend similar to that recorded before the administration of Sch. The eventual recovery of the heart's electrical activity was likely attributed to the natural metabolism of Sch in vivo. Overall, 13 out of total 16 rats in our experiments developed abnormal ECG patterns during our 25-min follow-up observation, and most of the events occurred in 10 min and last for at least 25 min after the Sch administration (Figure 3, B to D). In a significant contrast, abnormal cardiac electrical activity occurred in fewer rats (9 out of 16) and the duration of these unusual activity was much shorter when rats were treated with WP[6] at a dose of 20 mg/kg at 30 s after the administration of Sch, in comparison to those without WP[6] treatment (Figure 3, A to D). Even more significantly, abnormal ECG pattern only occurred in 2 out of 16 rats and the only two unusual hearts' electrical activity recovered back to a normal pattern in 25 min, when these rats were i.v. injected with WP[6] at the dose of 50 mg/kg at 30 s after the administration of Sch (Figure 3, A to D). These results clearly suggested that WP[6] may reverse the Sch induced cardiac arrhythmias in a dose-dependent manner.
WP[6] alleviated Sch induced hyperkalemia in a rat model. (A) The time dependent changes of serum potassium level of rats. Rats were i.v. administered with saline or WP[6] (20 mg/kg or 50 mg/kg) at 30s, 2 min and 5 min after Sch (0.9 mg/kg) administration. Group Ctrl and WP[6] 50 mg/kg, n = 4; other groups, n = 7. Data are presented as means ± SEM and *P < 0.05, **P < 0.01, ***P < 0.001. (B to E) Relative serum potassium level of rats at 5 min (B), 10 min (C), 15 min (D) and 25 min (E) after rats were i.v. administered with various samples. Data are presented as relative values and means ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001.
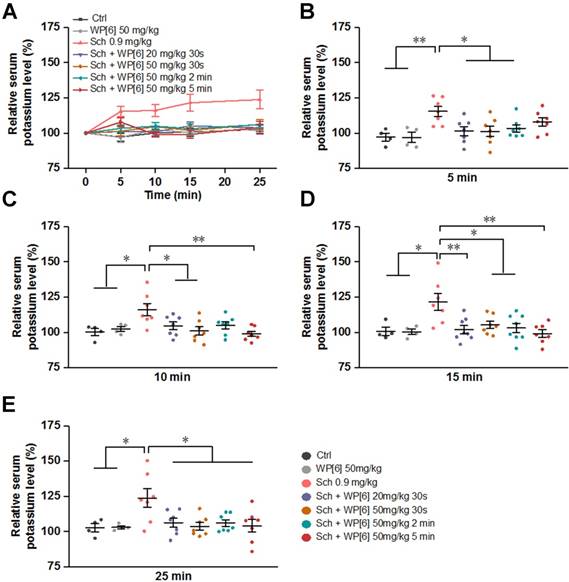
Alleviated Sch induced hyperkalemia in a rat model
In the current clinical practice, lethal hyperkalemia is one of the most deleterious side effects of Sch, especially for patients suffering from other medical conditions, such as muscle trauma and thermal trauma that up-regulate acetylcholine receptors [20, 63, 65, 66]. We therefore determined the serum potassium levels in rats that were treated with or without WP[6] after Sch administration. The serum potassium level increased continuously during 25-min follow up after rats were i.v. administered with Sch (0.9 mg/kg) (Figure 4, A to E). At 25 min time point, the mean serum potassium level was increased by about 25% in comparison to that determined before administration of Sch (Figure 4E). Conversely, when rats were treated with WP[6] (20 mg/kg or 50 mg/kg) at 30 s after administration of Sch, the mean serum potassium level in these rats kept relatively steady, exhibiting no significant differences when compared to those in the control group, at 5, 10, 15 and 25 min time points after the administration of Sch (Figure 4, A to E). Next, we investigated the serum potassium levels in rats in which WP[6] was administered at different time points after administration of Sch. Very interestingly, the relative serum potassium level did not change significantly at 5, 10, 15 and 25 min time points when rats were i.v. administered with WP[6] (50 mg/kg) at 2 min after administration of Sch. In another group, rats were then i.v. administered with WP[6] (50 mg/kg) at 5 min after administration of Sch, the already increased serum potassium level at 5 min time point gradually decreased down to the normal range at 10 min time point and maintained at the normal level at 15 and 25 min time points, comparable to those in the control group. This was likely attributed to the complexation and removal of Sch by WP[6] from AchRs, which halted efflux of intracellular potassium that was originally induced by Sch, and subsequent self-regulation of the organism. Taken together, WP[6] may effectively reverse the hyperkalemia induced by Sch in rats.
WP[6] alleviated the Sch induced rhabdomyolysis in a rat model. (A to C) The levels of creatine kinase (A), UA (B) and Crea (C) in the serum collected from rats sacrificed at 15 min after injected (via the right anterior tibial muscle) with saline, Sch (1 mg/kg), WP[6] (20 mg/kg or 50mg/kg at 30 s after administration of Sch) and WP[6] (50 mg/kg). n = 6 for each group; Data are presented as means ± SEM and *P < 0.05, **P < 0.01. (D) H&E histopathological analysis of the right anterior tibial muscle from rats sacrificed at 15 min after injected with saline, Sch (1 mg/kg), WP[6] (20 mg/kg or 50 mg/kg at 30 s after administration of Sch) and WP[6] (50 mg/kg). Scale bar = 50 µm.
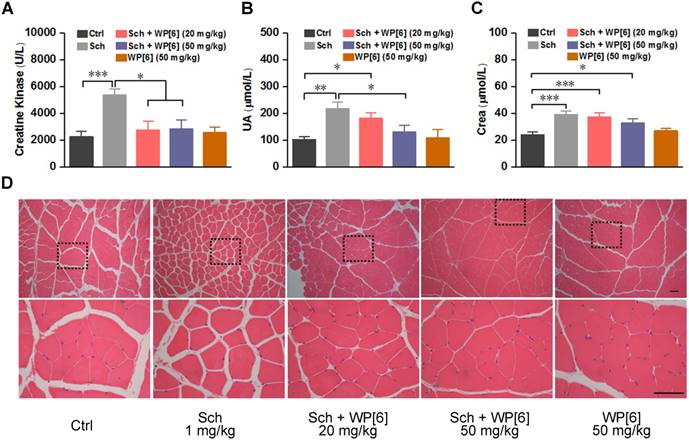
Alleviated Sch induced rhabdomyolysis in a rat model
Rhabdomyolysis may be induced by Sch in both adults and children [67-69]. This would result in loss of cellular contents such as electrolytes, myoglobin and creatine kinase, and severe rhabdomyolysis may further induce hyperkalemia and acute kidney injury [70, 71]. To test whether WP[6] could reduce the occurrence of rhabdomyolysis, rats were i.m. (via the right anterior tibia muscle) injected with WP[6] shortly after the i.m. administration of Sch. As shown in Figure 5A, creatine kinase, a serum biomarker of muscular damage [71, 72], increased by more than 2-fold at 15 min after rats were i.m. administered with Sch (1 mg/kg) [56]. In contrast, the level of creatine kinase was at the normal range when rats were treated with WP[6] (20 mg/kg and 50 mg/kg, respectively) at 30 s after administration of Sch, without significant difference with those of the rats in the control group. Similarly, the serum level of uric acid (UA) in the rats, the biomarker of renal function, was increased by about 2-fold at 15 min after administration with Sch (1 mg/kg), and it was slightly decreased when rats were treated with WP[6] at the dose of 20 mg/kg at 30 s after administration of Sch. When the treatment dose of WP[6] was 50 mg/kg, the serum level of UA showed no difference with the control group. (Figure 5B). The administration of WP[6] (20 mg/kg and 50 mg/kg, respectively) also moderately decreased the creatine level, although there were no statistical differences with those of the rats without WP[6] treatment (Figure 5C). The levels of urea and myoglobin in both untreated and treated groups of rats showed no differences (Figure S7). Furthermore, we performed hematoxylin and eosin (H&E) histopathological analysis of the right anterior tibia muscle collected at 15 min after the rats were treated with or without WP[6] at 30 s after the i.m. administration of Sch (1 mg/kg). In the control group that were administered with saline, the muscular cells in the fiber were compact with little intercellular space (Figure 5D). In contrast, the intercellular space of the muscular fibers became much wider when rats were i.m. administered with Sch (1 mg/kg), in line with the results reported previously [56]. And this severe condition was reversed to a large extent when rats were treated with WP[6] (either 20 mg/kg or 50 mg/kg) at 30 s after the i.m. administration of Sch (Figure 5D). These results suggested that WP[6] may effectively alleviate Sch induced rhabdomyolysis.
Reversal of paralysis in a mouse model
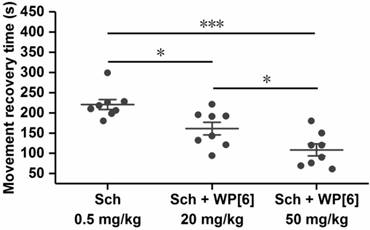
Undesired, prolonged paralysis might be induced by a single high dose or repeated dose of Sch, especially for patients with reduced cholinesterase activity [1, 73]. We therefore tested whether WP[6] could reverse the paralysis induced by Sch and accelerate mobility recovery in a mouse model. The paralysis was induced immediately after mice were i.v. administered with Sch (0.5 mg/kg) as described above, and these mice could not move on an accelerating rotor, and all of them fell off from the rotor immediately after they were placed there. Due to the metabolism of Sch in vivo, these mice gradually recovered from paralysis in several minutes and could move on the accelerating rotor continuously for more than 30 s (similar to the control mice), and the mean recovery time was about 226 s for the mice that were i.v. administered with Sch (0.5 mg/kg) (Figure 6). In contrast, the mean recovery time was decreased down to 157 s and 114 s respectively when mice were i.v. administered with WP[6] at 20 mg/kg and 50 mg/kg doses, respectively, at 30 s after the administration of Sch. This may be attributed to the complexation of Sch by WP[6] in the blood and the motor end-plate, causing a more rapid recovery of the neuromuscular activity. These findings suggested that WP[6] might be used to reverse Sch induced, undesirable, prolonged paralysis when Sch was misused or overdosed in clinics.
Reversed changes of plasma membrane potential and reduced loss of intracellular potassium in vitro
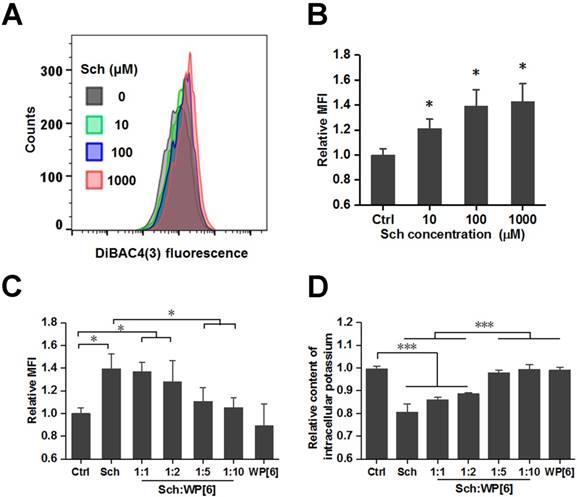
After having demonstrated that WP[6] could reverse Sch induced mortality and a variety of side effects including cardiac arrhythmia, hyperkalemia, rhabdomyolysis and prolonged paralysis in mammals including rat and mouse models, the reversal mechanism was subsequently investigated at a cellular level with L6 skeletal muscle cell line, which is well known to be susceptible to depolarization by Ach [74]. As a depolarizing NMBA, Sch may depolarize the plasma membrane of the muscle fiber and induce changes of plasma membrane potential, resulting in efflux of intracellular potassium [75]. A potential-sensitive fluorescent probe, Bis-(1,3-Dibutylbarbituric Acid)Trimethine Oxonol (DiBAC4(3)), was used to determine the relative plasma membrane potential, and the cellular fluorescence would be enhanced upon depolarization of the cells. As shown in Figure 7 A and B, the relative mean fluorescence intensity (MFI) of cells significantly increased during the addition of Sch. And the relative MFI was significantly reduced when cells were co-incubated with both Sch (100 µM) and WP[6] at the molar ratio of 1:5 and 1:10, respectively, when compared with that of the cells incubated with free Sch (Figure 7C and Figure S8). This indicated that Sch induced depolarization was reversed by WP[6] at the cellular level. Next, we examined whether WP[6] could reverse Sch induced efflux of intracellular potassium. The intracellular potassium was lost by about 20 % when cells were incubated with Sch (100 µM) for 10 min, and this condition was completely reversed when cells were co-incubated with both Sch and WP[6] (1:5 and 1:10, respectively) (Figure 7D). These results suggested that WP[6] could reverse Sch induced depolarization and reduce loss of the intracellular potassium.
Reversal of paralysis in a mouse model. Mice were i.v. administered with WP[6] (20 mg/kg or 50 mg/kg) at 30 s after the administration of Sch (0.5 mg/kg). n = 8 for each group, Data are presented as the original data and means ± SEM, *P < 0.05, ***P < 0.001.
WP[6] reversed the changes of plasma membrane potential and reduced the loss of intracellular potassium in vitro. (A and B) Typical flow cytometry curves (A) and quantitative analysis (B) for determination of the plasma membrane potential of L6 cells that were incubated with 0, 10, 100 and 1000 µM Sch for 10 min. MFI: mean fluorescence intensity. Data are presented as means ± SEM. n = 4, *P < 0.05. (C) Quantitative analysis for determination of the plasma membrane potential of L6 cells that were incubated with 100 µM Sch, WP[6] (1 mM) or co-incubated with Sch (100 µM) and WP[6] (molar ratio 1:1, 1:2, 1:5 and 1:10) for 10 min. Data are presented as means ± SEM. n = 4, *P < 0.05, *P < 0.01 and ***P < 0.001. (D) Relative content of intracellular potassium of L6 cells that were incubated with 100 µM Sch, WP[6] (1 mM) or co-incubated with Sch (100 µM) and WP[6] (molar ratio 1:1, 1:2, 1:5 and 1:10) for 10 min. Data are presented as means ± SEM. n = 4, ***P < 0.001.
Discussion
Sch was introduced into clinical practice as a muscle relaxant as early as in 1952, and has been used extensively during anesthesia ever since [76, 77]. With its increased use over time, a variety of severe side effects have been continuously reported [78, 79]. Therefore, researchers and anesthesiologists frequently turned to non-depolarizing NMBAs that exhibit relatively slower onset and longer action duration, but with less side effects than Sch. Several non-depolarizing NMBAs have been developed and widely used in clinical practice during past decades [1, 79]. However, their slow onset and longer action duration as well as potential cardiopulmonary side effects, have indeed limited their clinical use (Table S2). Among them, rocuronium has a relatively rapid onset of action (1-3 min) and its long neuromuscular blocking effect (30-70 min) could be reversed by cholinesterase inhibitors or sugammadex. In fact, the introduction of rocuronium initiated a debate in anesthesiology about who, between rocuronium and Sch, is the gold standard for RSI. Two clinical studies claimed that rocuronium could create the same optimal intubating conditions with those of Sch, after having analyzed the data from 50 and 401 patients, respectively [80, 81]. However, after reviewing total 50 trials including 4,151 patients, the Cochrane Collaboration concluded that Sch is superior to rocuronium for RSI, as Sch could achieve acceptable intubating conditions faster (by 30-60 s) than rocuronium, thus rocuronium should be used as an alternative only in cases where Sch is contraindicated [82]. Recently, another two new NMBAs (gantacurium and CW002) exhibit relatively rapid onset (90 s) and their neuromuscular blocking effect could be effectively reversed by cholinesterase inhibitors and cysteine [83], however they are not clinically approved yet and further studies need to be conducted to confirm their safety in humans [84]. Therefore, Sch still plays an irreplaceable role as a NMBA in situations that require RSI and is still clinically used extensively in anesthesia around the globe. For instance, Sch was used in 75% of RSI procedures in 18 emergency departments of United States, Canada and Australia from 2002 to 2012 [85]. 18 % of the hospitals used Sch for RSI in a survey comprising 266 hospitals in the UK in 2016 [86]. And this number was 62% in the emergency medical services in Germany in 2017 [87]. In Turkey, Sch was selected for adult elective cases (54.2%), pediatric elective cases (29.3 %), as well as the emergency cases (74.1%) [88]. Apart from its direct use for RSI, Sch is also used when rocuronium is effectively antagonized with Sugammadex but the neuromuscular blockage needs to be continued. In addition, Sch is used in electroconvulsive therapy, as the gold standard for the treatment of laryngospasm.
What causes the adverse effects of Sch? This has been extensively discussed and summarized in several reviews [78, 79]. The depolarization of motor nerve terminal, motor end-plate and muscle cell induced by Sch may result in fasciculation with increased abdominal pressure, myotonic response, potassium efflux (hyperkalemia), myalgia and rhabdomyolysis. Its agonistic actions at other nAchRs and mAchRs sites may induce tachycardia and hypertension, catecholamine release, sinus bradycardia and arrest, cardiac dysrhythmia, and increased intracranial/intraocular pressure. Sch may also induce phase Ⅱ depolarizing blockade and anaphylaxis. Sch's metabolites including succinylmonocholine and choline may augment these adverse conditions. Moreover, more severe side effects may get induced in patients with decreased plasma cholinesterase activities or increased AchRs. With the desperate medical needs, our studies, for the first time, suggest that WP[6] may scavenge Sch with a relatively high binding affinity, reverse depolarization of Sch in a cellular model and reduce Sch induced cardiac arrhythmia, hyperkalemia and rhabdomyolysis in rat models, accelerate recovery of paralysis and significantly improve survival rates of Sch-treated mice models.
The action mechanism of the macrocyclic molecules studied herein is reminiscent of that of Sugammadex and Calabadion [38-41], reversal agents of aminosteroids NMBAs. When injected into the circulatory system, WP[6] may specifically sequester Sch in the blood and neuromuscular junctions, reversing its depolarization and alleviating its neuromuscular blocking effects. Once the depolarization was reversed, Sch-induced paralysis, rhabdomyolysis, hyperkalemia and cardiac arrhythmia would be alleviated simultaneously. Of note, CB[7] binds Sch with a similar binding affinity to that between WP[6] and Sch, however WP[6] exhibited much better antidotal efficacy than CB[7] in reversal of the Sch-induced mortality. This might be attributed to nonspecific binding of CB[7] towards plasma proteins, peptides and amino acids in the blood, which may negatively influence the recognition and sequestration of Sch by CB[7] [89]. On the other hand, WP[5], which is more synthetically available, was also examined as a potential reversal agent for Sch. The binding affinity of WP[5] and SCh determined by ITC was 2.33×104 M-1 in water (Figure S9), which may be too week to effectively scavenge Sch from AchRs. Thus WP[5] was not further investigated in vivo in our studies.
Conclusion
In summary, several synthetic receptors, including WP[6], SC[4]A and CB[7], all of which exhibited high binding affinities with Sch, could reverse overdose Sch-induced mortality in mice models, in which WP[6] showed the best antidotal efficacy. Further studies showed that a single dose of WP[6] reversed paralysis in a mouse model and significantly alleviated a variety of side effects of Sch, including cardiac arrhythmia, hyperkalemia, rhabdomyolysis in rat models. At the cellular level, WP[6] reversed Sch-induced plasma membrane potential changes (depolarization) and efflux of intracellular potassium. This study strongly supports the development of macrocyclic receptors, particularly WP[6], as potential antidotes and supramolecular therapeutics to counteract the adverse effects of Sch that is used in millions of medical procedures annually around the globe.
Abbreviations
Ach: acetylcholine; AchRs: acetylcholine receptors; ALT: alanine aminotransferase; AST: aspartate aminotransferase; CB[7]: cucurbit[7]uril; Ch: choline; CLSM: confocal laser scanning microscopy; Crea: creatinine; DMEM: Dulbecco's modified eagle medium; DiBAC4(3): Bis-(1,3-Dibutylbarbituric Acid)Trimethine Oxonol; ECG: Electrocardiogram; FBS: fetal bovine serum; HBSS: Hank's Balanced salt solution; HGB: hemoglobin; H&E: hematoxylin-eosin; GA: genetic algorithm; ITC: isothermal titration calorimetry; LGA: lamarckian genetic algorithm; mAchRs: muscarinic acetylcholine receptors; nAchRs: nicotine acetylcholine receptors; NMBA: neuromuscular blocking agent; PLT: platelet; PBS: phosphate-buffered saline; RBC: red blood cell; RSI: rapid sequence induction; SC[4]A: sulfo-calixar[4]ene; Sch: Succinylcholine; UA: uric acid; WBC: whole blood cell; WP[6]: water-soluble carboxylotepillar[6]arene.
Supplementary Material
Supplementary figures and tables.
Acknowledgements
Financial support
This work was financially supported by Macau Science and Technology Development Fund (Grant No.: FDCT 030/2017/A1 and FDCT 0121/2018/A3), the Research Committee at the University of Macau (Grant No.: MYRG2016-00008-ICMS-QRCM, MYRG2016-00165-ICMS-QRCM, and MYRG2017-00010-ICMS), and the National Science Foundation of China (Grant No.: 21871301). We are also grateful to Prof. Leyong Wang and Prof. Dongsheng Guo for providing technical support to the project.
Author contributions
R. W., J. Z., and X. Z. conceived the project, with F. H. assisting in the study design. R. W., J. Z., and X. Z. designed studies and conducted data analysis. X. Z. performed most of the experiments. Q. C. helped performing ITC experiments. L. L. and C. L. advised on animal studies. L. S. and S. L. helped synthesizing WP[6]. X. Z., R. W. and J. Z. wrote the manuscript and all authors edited the final manuscript.
Competing Interests
X.Z. and R.W. are in the process of filing a patent relating to the content of this work. All other authors have no competing interest to declare.
References
1. Appiah-Ankam J, Hunter JM. Pharmacology of neuromuscular blocking drugs. BJA Educ. 2004;4:2-7
2. Baraka A. Succinylcholine "the gold standard" for rapid-sequence induction of anesthesia. Middle East J Anesthesiol. 2011;21:323
3. Al-alami AA, Zestos MM, Baraka AS. Pediatric laryngospasm: prevention and treatment. Curr Opin Anesthesiol. 2009;22:388-95
4. Bryson EO, Aloysi AS, Popeo DM, Bodian CA, Pasculli RM, Briggs MC. et al. Methohexital and succinylcholine dosing for electroconvulsive therapy (ECT): actual versus ideal. J ECT. 2012;28:e29-e30
5. Li EH, Bryson EO, Kellner CH. Muscle relaxation with succinylcholine in electroconvulsive therapy. Anesth Analg. 2016;123:1329
6. Bauld HW, Gibson PF, Jebson PJ, Brown SS. Etiology of prolonged apnea after suxamethonium. Br J Anaesth. 1974;46:273-81
7. Wong S, Chung F. Succinylcholine-associated postoperative myalgia. Anaesthesia. 2000;55:144-52
8. Schreiber J-U, Lysakowski C, Fuchs-Buder T, Tramer MR. Prevention of succinylcholine-induced fasciculation and myalgiaa meta-analysis of randomized trials. Anesthesiology. 2005;103:877-84
9. Miller RD, Way WL, Hickey RF. Inhibition of succinylcholine-induced increased intraocular pressure by non-depolarizing muscle relaxants. Anesthesiology. 1968;29:123-5
10. Kelly RE, Dinner M, Turner LS, Haik B, Abramson DH, Daines P. Succinylcholine increases intraocular pressure in the human eye with the extraocular muscles detached. Anesthesiology. 1993;79:948-52
11. Martyn JAJ, Richtsfeld M. Succinylcholine-induced hyperkalemia in acquired pathologic statesetiologic factors and molecular mechanisms. Anesthesiology. 2006;104:158-69
12. Genever E. Suxamethonium-induced cardiac arrest in unsuspected pseudohypertrophic muscular dystrophy. Br J Anaesth. 1971;43:984-6
13. Sullivan M, Thompson WK, Hill GD. Succinylcholine-induced cardiac arrest in children with undiagnosed myopathy. Can J Anaesth. 1994;41:497-501
14. Smith RB. Cardiac arrhythmias following the administration of succinylcholine. Anesth Prog. 1971;18:9
15. Dexter F, Epstein RH, Wachtel RE, Rosenberg H. Estimate of the relative risk of succinylcholine for triggering malignant hyperthermia. Anesth Analg. 2013;116:118-22
16. Hansen D. Suxamethonium-induced cardiac arrest and death following 5 days of immobilization. Eur J Anaesthesiol. 1998;15:240-1
17. Verma A, Bedlack RS, Radtke RA, VanLandingham KE, Erwin CW. Succinylcholine induced hyperkalemia and cardiac arrest: Death related to an EEG study. J Clin Neurophysiol. 1999;16:46-50
18. Gronert GA. Cardiac arrest after succinylcholine: mortality greater with rhabdomyolysis than receptor upregulation. Anesthesiology. 2001;94:523-9
19. Patanwala AE, Erstad BL, Roe DJ, Sakles JC. Succinylcholine is associated with increased mortality when used for rapid sequence intubation of severely brain injured patients in the emergency department. Pharmacotherapy. 2016;36:57-63
20. Martyn AJ, Richtsfeld M. Succinylcholine-induced hyperkalemia in acquired pathologic statesetiologic factors and molecular mechanisms. Anesthesiology. 2006;104:158-69
21. Lehnhardt A, Kemper MJ. Pathogenesis, diagnosis and management of hyperkalemia. Pediatr Nephrol. 2011;26:377-84
22. Krause T, Gerbershagen MU, Fiege M, Weißhorn R, Wappler F. Dantrolene - a review of its pharmacology, therapeutic use and new developments. Anaesthesia. 2004;59:364-73
23. Alok S, Hemangi K, Sanjay K, Kanchan J. Malignant hyperthermia: dantrolene sodium - a must have. Indian J Anaesth. 2012;56:212-3
24. Larach MG, Klumpner TT, Brandom BW, Vaughn MT, Belani KG, Herlich A. et al. Succinylcholine use and dantrolene availability for malignant hyperthermia treatment: database analyses and systematic review. Anesthesiology. 2019;130:41-54
25. Lehn J-M. Supramolecular chemistry—scope and perspectives molecules, supermolecules, and molecular devices (nobel lecture). Angew Chem. 1988;27:89-112
26. Ma X, Zhao Y. Biomedical applications of supramolecular systems based on host-guest interactions. Chem Rev. 2015;115:7794-839
27. Kolesnichenko IV, Anslyn EV. Practical applications of supramolecular chemistry. Chem Soc Rev. 2017;46:2385-90
28. Guo D-S, Liu Y. Suprannolecular chemistry of p-sulfonatocalix[n]arenes and its biological applications. Acc Chem Res. 2014;47:1925-34
29. Song N, Kakuta T, Yamagishi T-a, Yang Y-W, Ogoshi T. Molecular-scale porous materials based on pillarnarenes. Chem. 2018;4:2029-53
30. Yu Q, Zhang Y-M, Liu Y-H, Xu X, Liu Y. Magnetism and photo dual-controlled supramolecular assembly for suppression of tumor invasion and metastasis. Sci Adv. 2018;4:eaat2297
31. Lou X-Y, Li Y-P, Yang Y-W. Gated materials: Installing macrocyclic arenes-based supramolecular nanovalves on porous nanomaterials for controlled cargo release. Biotechnol J. 2019:14
32. Mattia E, Otto S. Supramolecular systems chemistry. Nat Nanotechnol. 2015;10:111
33. Li S, Chan JY-W, Li Y, Bardelang D, Zheng J, Yew WW. et al. Complexation of clofazimine by macrocyclic cucurbit [7] uril reduced its cardiotoxicity without affecting the antimycobacterial efficacy. Org Biomol Chem. 2016;14:7563-9
34. Yang X, Wang Z, Niu Y, Chen X, Lee SM, Wang R. Influence of supramolecular encapsulation of camptothecin by cucurbit[7]uril: reduced toxicity and preserved anti-cancer activity. Medchemcomm. 2016;7:1392-7
35. Yu G, Chen X. Host-guest chemistry in supramolecular theranostics. Theranostics. 2019;9(11):3041-3074 doi:10.7150/thno.31653
36. Shields M, Giovannelli M, Mirakhur R, Moppett I, Adams J, Hermens Y. Org 25969 (sugammadex), a selective relaxant binding agent for antagonism of prolonged rocuronium-induced neuromuscular block. Br J Anaesth. 2006;96:36-43
37. Bom A, Bradley M, Cameron K, Clark JK, van Egmond J, Feilden H. et al. A novel concept of reversing neuromuscular block: Chemical encapsulation of rocuronium bromide by a cyclodextrin-based synthetic host. Angew Chem. 2002;41:265-70
38. Ma D, Zhang B, Hoffmann U, Sundrup MG, Eikermann M, Isaacs L. Acyclic cucurbit[n]uril-type molecular containers bind neuromuscular blocking agents in vitro and reverse neuromuscular block in vivo. Angew Chem. 2012;124:11520-4
39. Hoffmann U, Grosse-Sundrup M, Eikermann-Haerter K, Zaremba S, Ayata C, Zhang B. et al. Calabadion: a new agent to reverse the effects of benzylisoquinoline and steroidal neuromuscular-blocking agents. Anesthesiology. 2013;119:317-25
40. Haerter F, Simons JCP, Foerster U, Moreno Duarte I, Diaz-Gil D, Ganapati S. et al. Comparative effectiveness of calabadion and sugammadex to reverse non-depolarizing neuromuscular-blocking agents. Anesthesiology. 2015;123:1337-49
41. Diaz-Gil D, Haerter F, Falcinelli S, Ganapati S, Hettiarachchi GK, Simons JCP. et al. A novel strategy to reverse general anesthesia by scavenging with the acyclic cucurbit[n]uril-type molecular container calabadion 2. Anesthesiology. 2016;125:333-45
42. Wyman IW, Macartney DH. Host-guest complexes and pseudorotaxanes of cucurbit[7]uril with acetylcholinesterase inhibitors. J Org Chem. 2009;74:8031-8
43. Abd El-Rahman MK, Mahmoud AM. A novel approach for spectrophotometric determination of succinylcholine in pharmaceutical formulation via host-guest complexation with water-soluble p-sulfonatocalixarene. RSC Adv. 2015;5:62469-76
44. Coleman AW, Jebors S, Cecillon S, Perret P, Garin D, Marti-Battle D. et al. Toxicity and biodistribution of para-sulfonato-calix[4]arene in mice. New J Chem. 2008;32:780-2
45. Zhang X, Xu X, Li S, Wang L-H, Zhang J, Wang R. A systematic evaluation of the biocompatibility of cucurbit[7]uril in mice. Sci Rep. 2018;8:8819
46. Yu G, Xue M, Zhang Z, Li J, Han C, Huang F. A water-soluble pillar[6]arene: synthesis, host-guest chemistry, and its application in dispersion of multiwalled carbon nanotubes in water. J Am Chem Soc. 2012;134:13248-51
47. Yu G, Zhou X, Zhang Z, Han C, Mao Z, Gao C. et al. Pillar[6]arene/paraquat molecular recognition in water: high binding strength, ph-responsiveness, and application in controllable self-assembly, controlled release, and treatment of paraquat poisoning. J Am Chem Soc. 2012;134:19489-97
48. Shu X, Xu K, Hou D, Li C. Molecular recognition of water-soluble pillar[n]arenes towards biomolecules and drugs. Isr J Chem. 2018;58:1230-40
49. Yang K, Pei Y, Wen J, Pei Z. Recent advances in pillar[n]arenes: synthesis and applications based on host-guest interactions. Chem Commun. 2016;52:9316-26
50. Feng W, Jin M, Yang K, Pei Y, Pei Z. Supramolecular delivery systems based on pillararenes. Chem Commun. 2018;54:13626-40
51. Song N, Lou X-Y, Ma L, Gao H, Yang Y-W. Supramolecular nanotheranostics based on pillarenes. Theranostics. 2019;9(11):3075-3093 doi:10.7150/thno.31858
52. Day A, Arnold AP, Blanch RJ, Snushall B. Controlling factors in the synthesis of cucurbituril and its homologues. J Org Chem. 2001;66:8094-100
53. Kim J, Jung I-S, Kim S-Y, Lee E, Kang J-K, Sakamoto S. et al. New cucurbituril homologues: syntheses, isolation, characterization, and X-ray crystal structures of cucurbit [n] uril (n= 5, 7, and 8). J Am Chem Soc. 2000;122:540-1
54. Bardelang D, Udachin KA, Leek DM, Margeson JC, Chan G, Ratcliffe CI. et al. Cucurbit[n]urils (n= 5-8): a comprehensive solid state study. Cryst Growth Des. 2011;11:5598-614
55. Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. 2010;31:455-61
56. Stambolija V, Stambolija TP, Holjevac JK, Murselovic T, Radonic J, Duzel V. et al. BPC 157: the counteraction of succinylcholine, hyperkalemia, and arrhythmias. Eur J Pharmacol. 2016;781:83-91
57. Hou Vivian Y, Hirshman Carol A, Emala Charles W. Neuromuscular relaxants as antagonists for M2 and M3 muscarinic receptors. Anesthesiology. 1998;88:744-50
58. Borea PA, Varani K, Gessi S, Gilli P, Gilli G. Binding thermodynamics at the human neuronal nicotine receptor. Biochem Pharmacol. 1998;55:1189-97
59. Jonsson M, Dabrowski M, Gurley David A, Larsson O, Johnson Edwin C, Fredholm Bertil B. et al. Activation and inhibition of human muscular and neuronal nicotinic acetylcholine receptors by succinylcholine. Anesthesiology. 2006;104:724-33
60. Fagerlund Malin J, Dabrowski M, Eriksson Lars I. Pharmacological characteristics of the inhibition of nondepolarizing neuromuscular blocking agents at human adult muscle nicotinic acetylcholine receptor. Anesthesiology. 2009;110:1244-52
61. Sanchez CJ, Hooper E, Garry PJ, Goodwin JM, Goodwin JS. The relationship between dietary intake of choline, choline serum levels, and cognitive function in healthy elderly persons. J Am Geriatr Soc. 1984;32:208-12
62. Williams RE, Gain EN, Hunter AR. Electrocardiographic changes following repented injections of decamethonium and subsequent injections of succinylcholine. Sur Anesth. 1964;8:2
63. Evers W, Racz GB, Dobkin AB. A study of plasma potassium and electrocardiographic changes after a single dose of succinylcholine. Can J Anaesth. 1969;16:273-81
64. Staikou C, Stamelos M, Stavroulakis E. Impact of anaesthetic drugs and adjuvants on ECG markers of torsadogenicity. Br J Anaesth. 2014;112:217-30
65. Martyn Jeevendra JA. Succinylcholine hyperkalemia after burns. Anesthesiology. 1999;91:321-2
66. Gronert Gerald A. Succinylcholine-induced hyperkalemia and beyond. Anesthesiology. 2009;111:1372-7
67. Lewandowski KB. Rhabdomyolysis, myoglobinuria and hyperpyrexia caused by suxamethonium in a child with increased serum creatine kinase concentrations. Br J Anaesth. 1981;53:981-4
68. Friedman S, Baker T, Gatti M, Simon G, Paskin S. Probable succinylcholine-induced rhabdomyolysis in a male athlete. Anesth Analg. 1995;81:422-3
69. Gronert Gerald A. Cardiac arrest after succinylcholinemortality greater with rhabdomyolysis than receptor upregulation. Anesthesiology. 2001;94:523-9
70. Bosch X, Poch E, Grau JM. Rhabdomyolysis and acute kidney injury. N Engl J Med. 2009;361:62-72
71. Chavez LO, Leon M, Einav S, Varon J. Beyond muscle destruction: a systematic review of rhabdomyolysis for clinical practice. Crit Care. 2016;20:135
72. Brancaccio P, Lippi G, Maffulli N. Biochemical markers of muscular damage. Clin Chem Lab Med. 2010;48:757-67
73. Levano S, Ginz H, Siegemund M, Filipovic M, Voronkov E, Urwyler A. et al. Genotyping the butyrylcholinesterase in patients with prolonged neuromuscular block after succinylcholine. Anesthesiology. 2005;102:531-5
74. Steinbach J. Acetylcholine responses on clonal myogenic cells in vitro. J Physiol. 1975;247:393-405
75. Thesleff S. The effects of acetyleholine, decamethonium and succinylcholine on neuromuscular transmission in the rat. Acta Physiol Scand. 1955;34:386-92
76. Bourne JG, Collier HOJ, Somers GF. Succinylcholine (succinoylcholine) muscle-relaxant of short action. The Lancet. 1952;259:1225-9
77. Foldes FF, Mcnall PG, Borrego-Hinojosa JM. Succinylcholine: a new approach to muscular relaxation in anesthesiology. N Engl J Med. 1952;247:596-600
78. Lee C. Suxamethonium in its fifth decade. Baillière's Clin Anaesth. 1994;8:417-40
79. Lee C. Goodbye suxamethonium!. Anaesthesia. 2009;64:73-81
80. Magorian T, Flannery KB, Miller RD. Comparison of rocuronium, succinylcholine, and vecuronium for rapid-sequence induction of anesthesia in adult patients. Anesthesiology. 1993;79:913
81. Marsch SC, Steiner L, Bucher E, Pargger H, Schumann M, Aebi T. et al. Succinylcholine versus rocuronium for rapid sequence intubation in intensive care: a prospective, randomized controlled trial. Crit Care. 2011;15:R199
82. Tran DTT, Newton EK, Mount VAH, Lee JS, Wells GA, Perry JJ. Rocuronium versus succinylcholine for rapid sequence induction intubation. Cochrane Database Syst Rev. 2015
83. Lien CA. Development and potential clinical impact of ultra-short acting neuromuscular blocking agents. Br J Anaesth. 2011;107:i60-i71
84. De Boer HD, Carlos RV. New drug developments for neuromuscular blockade and reversal: Gantacurium, cw002, cw011, and calabadion. Curr Anesth Rep. 2018;8:119-24
85. Brown CA, Bair AE, Pallin DJ, Walls RM. Techniques, success, and adverse events of emergency department adult intubations. Ann Emerg Med. 2015;65:363-70
86. Sajayan A, Wicker J, Ungureanu N, Mendonca C, Kimani PK. Current practice of rapid sequence induction of anaesthesia in the UK - a national survey. Br J Anaesth. 2016;117:i69-i74
87. Warnecke T, Dobbermann M, Becker T, Bernhard M, Hinkelbein J. Performance of prehospital emergency anesthesia and airway management: an online survey. Der Anaesthesist. 2018;67:654-63
88. Ömür D, Kiraz HA, Şahin H, Toman H, Uyan B, Ekin S. et al. Use of succinylcholine by anaesthetists in turkey: a national survey. Turkish J Anaesthesiol Reanim. 2015;43:323
89. Urbach AR, Ramalingam V. Molecular recognition of amino acids, peptides, and proteins by cucurbit[n]uril receptors. Isr J Chem. 2011;51:664-78
Author contact
![]() Corresponding author: Ruibing Wang, Jianxiang Zhang or Feihe Huang. Email: rwangedu.mo (Ruibing Wang); jxzhangedu.cn (Jianxiang Zhang); fhuangedu.cn (Feihe Huang).
Corresponding author: Ruibing Wang, Jianxiang Zhang or Feihe Huang. Email: rwangedu.mo (Ruibing Wang); jxzhangedu.cn (Jianxiang Zhang); fhuangedu.cn (Feihe Huang).
 Global reach, higher impact
Global reach, higher impact