13.3
Impact Factor
Theranostics 2019; 9(11):3094-3106. doi:10.7150/thno.31914 This issue Cite
Review
Gene delivery based on macrocyclic amphiphiles
1. College of Chemistry, Key Laboratory of Functional Polymer Materials (Ministry of Education), State Key Laboratory of Elemento-Organic Chemistry, Nankai University, Tianjin 300071, China.
2. State Key Laboratory of Quality Research in Chinese Medicine, Institute of Chinese Medical Sciences, University of Macau, Taipa, Macau SAR, China.
Received 2018-11-30; Accepted 2019-3-6; Published 2019-5-18
Abstract

Gene therapy, with an important role in biomedicine, often requires vectors for gene condensation in order to avoid degradation, improve membrane permeation, and achieve targeted delivery. Macrocyclic molecules are a family of artificial receptors that can selectively bind a variety of guest species. Amphiphilic macrocycles, particularly those bearing cationic charges and their various assemblies represent a new class of promising non-viral vectors with intrinsic advantages in gene condensation and delivery. The most prominent examples include amphiphilic cyclodextrins, calixarenes and pillararenes. Herein, we systemically reviewed reported assemblies of amphiphilic macrocycles for gene delivery and therapy. The advantages and disadvantages of each type of macrocyclic amphiphiles for gene delivery, as well as the perspectives on the future development of this area are discussed.
Keywords: macrocyclic amphiphiles, gene delivery
Introduction
Gene delivery is a process of introducing foreign DNA or RNA into host cells, which occurs in nature as horizontal gene transfer from one organism to another and is a mechanism of evolution [1, 2]. Controlled and efficient delivery of genes into living cells is important for both fundamental research and biomedical applications [3-5]. Naked DNA and RNA exhibit very poor capacity to transfect cells, because of a number of barriers and their decomposition by nucleases in biological media [6-8]. Indeed, DNA should first reach and bind the target cells, subsequently enter the cytoplasm either by crossing the cell plasma membrane or by escaping from the endosomes and lysosomes, and finally enter the nucleus. Therefore, efficient gene delivery requires specially designed nucleic acid vehicles-gene delivery vectors.
Viruses are able to achieve efficient gene delivery in a very competent manner, and a number of viral vectors have been successfully progressed to clinical trials and, in a few cases, to the market [9, 10]. Nevertheless, the concerns associated to their intrinsic immunogenicity, high production costs and limitations regarding the size of the polynucleotide that can be transported have limited their clinical applications. Alternatively, significant efforts have been made by chemists to develop artificial gene carriers, so-called non-viral vectors. The most traditional non-viral vectors are based on cationic lipids and polymers [11]. As a natural evolution, a large number of gene delivery systems that utilize nanoscale building blocks were proposed based on dendrimers [12, 13], gold nanoparticles (NPs) [14], silica NPs [15], polymer NPs [16-19], supramolecular NPs [20], upconversion NPs [21], and core-shell organic NPs [22].
Macrocyclic molecules, typically including crown ether [23], cyclodextrin (CD) [24-27], calixarene (CA) [28-30], cucurbituril [31-33], pillararene (PA) [34-37], and others [38, 39], are a family of well-studied artificial receptors with a discrete cavity that is selective for binding certain guests. Macrocyclic amphiphiles were often obtained by decorating hydrophilic groups at one side and hydrophobic groups at the opposite side of the macrocyclic scaffold [40]. From the viewpoint of structural characteristics, they incorporate both bola-type and gemini-type amphiphiles into a single molecule. More importantly, the unique superiority of macrocyclic amphiphiles is the reversible host-guest recognition. Macrocyclic amphiphiles were deemed as “surfactants with host-guest recognition sites” [41], in which cavities are distributed on the surface of the assembles. Macrocyclic amphiphiles have been widely applied in many fields such as biosensing [42-44], bioimaging [45], luminescent material [46-49], and drug delivery [50]. Notably, benefiting from the dual features of molecular recognition and self-assembly, macrocyclic amphiphiles have been increasingly investigated as a new family of gene delivery vectors. In this review, we summarize macrocyclic amphiphiles and their potential applications for gene delivery and therapy. There are much more works of gene delivery related to macrocycles, but we strategically focus on macrocyclic amphiphiles in this review. Based on different macrocyclic amphiphiles designed and studied for gene delivery, this review is divided into three sections, amphiphilic CDs, amphiphilic CAs, and amphiphilic PAs, attributed to the distinguished sizes, shapes and recognition abilities of individual type of macrocycles. Furthermore, we will discuss the pros and cons of the use of macrocyclic amphiphiles as gene delivery vectors, and provide perspectives for future development. Our targeted audience include scientists from the fields of supramolecular chemistry, gene therapy, biomaterials, pharmaceutical sciences, and nanotechnology.
Amphiphilic CDs for gene delivery
CDs are cyclic oligosaccharides consisting of D-glucose units linked by α-(1,4)-glucose bonds [51]. The most common CDs are α-, β-, and γ-CDs with 6, 7, and 8 glucose units, respectively. They are cylinder shaped and have hydrophobic inner cavity and hydrophilic outer surface. Therefore, they can complex with a variety of hydrophobic molecules [52]. On account of the intrinsic water-solubility of CDs, amphiphilic CDs could be obtained by grafting hydrophobic chains on the narrow (or wide) side, where introducing hydrophilic groups on the other side is not necessary. Given the negatively charged nature of DNA or RNA, a variety of amphiphilic CDs with simple cationic groups and with saccharide targeting groups (Figure 1) have been designed and investigated for efficient gene delivery.
Amphiphilic CDs modified with cationic groups
In 2004, O'Driscoll et al. reported amphiphilic CDs 1-4 with the hydrophobic and hydrophilic groups at the C-6 and C-2 positions, respectively [53]. In water, long-chained 2 and 4 formed vesicles, while short-chained 1 and 3 formed micelles. As expected, cationic 3 and 4 could bind to DNA, while neutral 1 and 2 couldn't. Transfection experiments in vitro revealed that, under optimum conditions, 3 and 4 exhibited decent transfection efficiencies that were comparable with that of the widely used transfection agent Lipofectin.
In 2012, the same research group synthesized structurally-similar amphiphilic CDs 5 and 6, both of which bear cationic charges [54, 55]. Very interestingly, the potential of these carriers for delivering small interfering RNA (siRNA) against neuropsychiatric and neurodegenerative disorders was demonstrated in human fibroblasts isolated from patients with Huntington's disease, and in the R6/2 mouse model of Huntington's disease [54]. Of note, in the in vivo studies, the co-assembly of 5 and a siRNA that can silence the Huntingtin toxic protein was directly injected into the mouse brain. Moreover, 5 and 6 were co-formulated to improve the physiochemical properties (i.e. lower surface charges and reduced tendency for aggregation) of the NPs with siRNA and excellent gene transfection was achieved in vitro [55]. More interestingly, DSPE-PEG-Fab was linked onto the NPs formulated between 5 and siRNA via a “postinsertion” approach enabled delivering siRNA targeting antibody to related to acute myeloid leukemia (leukemia stem cells) [56].
In 2009, they developed another series of amphiphilic CDs 7-15 by selectively alkylating the more acidic O-2 hydroxyls, and decorating the primary C-6 positions with cationic arms [57]. Among these CDs, 9 can deliver DNA with a comparable transfection efficiency to polyethylenimine (PEI), one of the most efficient commercial gene delivery systems, into undifferentiated and differentiated Caco-2 (human colon adenocarcinoma) cells [58]. The complex formed by 13/DNA was resistant to pancreatic enzymes lipase, amylase and protease and offered some protection against DNase. This assembly exhibited significant potential as an intestinal delivery vector, attributed to its superior stability and enhanced the transfection efficiency in the presence of bile salt [59].
Fernández et al. reported another group of polycationic amphiphilic CDs 16-48 with amino groups at one side and alkyl chains or aromatic substituents at the other side [60-67]. Among them, 18 and 19, both of which possess a long aliphatic chain (13 carbons), have much higher transfection efficiencies than 16 and 17, which contain a relatively shorter aliphatic chain (5 carbons) on the lipophilic side, suggesting the importance of high hydrophobicity in the amphiphilic structures for delivering plasmid DNA. Moreover, the self-assembled CDs/DNA NPs prepared from 24 and 30 exhibited transfection efficiencies higher than PEI/DNA polyplexes. The complexes of mRNA or pDNA with 27 can lead to efficient protein expression in vitro and in vivo [68], but the transfection efficiency drastically decreased after incubation with ascites fluid [69]. Compounds 21, 30 and 37 can complex with pDNA and promote transfection in both Hela and MCF-7 cells. Interestingly, coating of protein corona on these vector's surfaces, in the presence of human serum, did not impact pDNA delivery and transfection [70]. In addition, compound 38 was demonstrated to effectively protect siRNA from degradation by RNAses, and the thermodynamic parameters and the transfection in several cell lines by 38/siRNA were investigated thoroughly, showing the significant potential of 38 as a siRNA delivery vector [71].
Molecular structures of amphiphilic CDs employed in gene delivery.
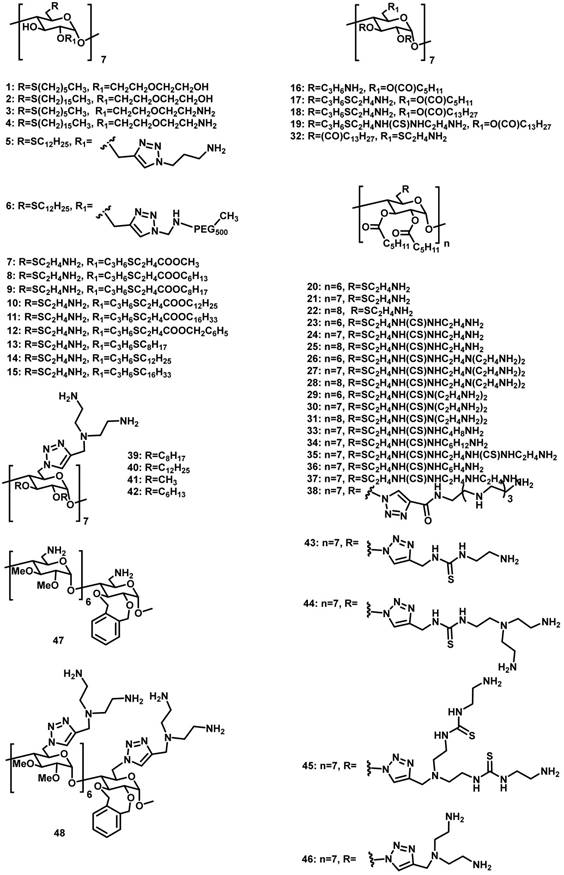
Based on this series of works, some general conclusions were obtained: (1) β-CD derivatives are overall superior to their α-CD and γ-CD homologues, which probably because they can complex with cholesterol, and thereby have higher biological membrane permeability [62]; (2) a dendritic structure is more favorable than a linear homologues by comparing a variety of amphiphilic CDs with the same number of amino groups (branched vs linear); (3) increasing the number of ethyleneimine segments per branch resulted in better transfection performances; (4) incorporating thiourea segments enhanced the transfection capabilities, likely due to formation of more hydrogen bonds with the polyphosphate backbone; (5) the hydrophilic/hydrophobic balance is important to efficient self-assembly in the presence of DNA; (6) these vectors internalized through clathrin- and caveolin-dependent endocytosis, but the later route is dominant regarding transfection [63]; (7) In vivo, transfection occurred mainly in the liver and partially in the lungs, therefore these vectors may be well-suited for cytokine-based hepatocellular carcinoma.
In 2013, Ilarduya et al. treated 30/DNA complex with folic acid (FA) and obtained ternary systems, in which the FA units were anchored at the periphery of NPs through electrostatic interactions with CDs [72]. The FA modified nanocomplex efficiently promoted transfection in HeLa cells without associated toxicity. It was further demonstrated as an efficient vector for gene delivery in vivo in a mouse model, showing relatively high transfection levels in both the lung and, the liver.
Amphiphilic CDs with saccharide targeting groups
Introducing saccharide groups on the amphiphilic CD vectors may offer excellent opportunities for targeted delivery of genes. In 2011, Fernández et al. reported mannosylated amphiphilic CD 49 [73]. The self-assembled NPs obtained from these mannosylated CDs and DNA can be specifically recognized by mannose-specific lectins, including concanavalin A and macrophage mannose receptor. Moreover, 49/DNA complex showed transfection in RAW 264.7 cells that is well known to overexpress the macrophage mannose receptor, but showed no transfection in BNL-CL2 cells that do not express mannose receptors. Macrophage adhesion experiments also indicated the existence of unspecific binding, probably because of electrostatic interactions of NPs with negatively charged cell membrane components. The relative specific versus non-specific internalization was dependent on the CD/DNA proportion.
In 2012, the same research group reported amphiphilic CDs derivatized with aminoglucoside 50-52 or with galactosyl motifs 53-54 [74, 75]. Compounds 50 and 52 can efficiently promote cellular uptake and subsequent gene expression in COS-7 cells. 53 and 54 were selectively recognized by the asialoglycoprotein receptor at the surface of hepatocytes, and were efficiently endocytosed by HepG2 or BNL-CL2 cells. However, the clathrin-mediated internalization route operating in this case did not result in efficient DNA transfection, probably because endosomal escape occurred at a very early stage and the gene material was not trafficked to the vicinity of the nucleus. Interestingly, when DNA was replaced by a messenger RNA, which does not need to reach the nucleus to exert its biological function, the transfection performances became much higher than that of the commercial vector JetPEI-Hep, suggesting a strong potential of the vectors in mRNA-based gene therapy.
In 2012, O'Driscoll et al. reported amphiphilic CDs 55-60 bearing galactose-targeting ligands with different linker lengths and co-formulated with cationic amphiphilic CD vectors [76]. Recognition of the targeting ligands on CDs by a galactose-specific lectin was demonstrated in vitro. Interestingly, the binding affinities decreased with increased linker length between the galactosyl group and the CD core. However, contrary to the lectin binding results, transfection levels increased with an increase in linker length from 7 to 15 carbon atoms. In comparison with the formulations without targeting molecules, a significant increase in transfection efficiency was observed only in the presence of dioleoylphosphatidylethanolamine (DOPE).
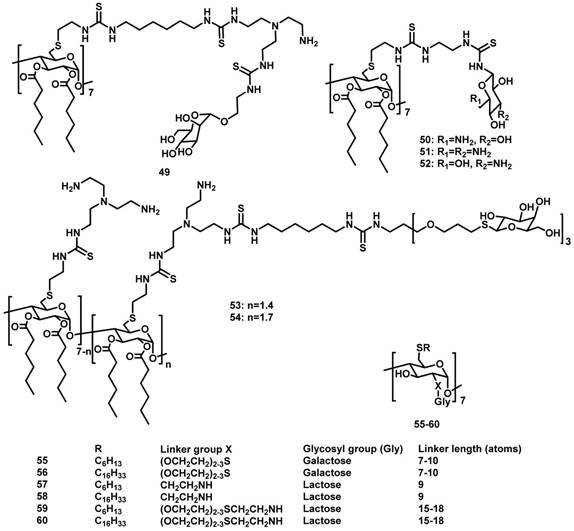
Amphiphilic CAs for gene delivery
CAs are composed of phenolic units bridged with methylene groups at o-positions of hydroxyl groups [77]. CAs with n phenolic units are often referred to as calix[n]arenes and the most popular CAs are those with n = 4, 5, 6, and 8. CAs, once described as having “(almost) unlimited possibilities” because of their facile modification [78, 79], represent a popular macrocyclic scaffold in construction of versatile nanostructures. Of note, CAs and related macrocycles carrying a variety of functional groups, such as glycol-, amino-, polyamino-, guanidino-, and imidazo-moieties, were previously reviewed back in 2014 for their potential applications in gene delivery [80]. In this section we exclusively focus on the amphiphilic CAs that have been explored up to early 2019. The upper and lower rims of CA could be easily functionalized with different hydrophilicity/hydrophobicity, which leads to generation of amphiphilic CAs. A variety of supramolecular assemblies of amphiphilic CAs including micelles and vesicles have been used in many fields [81, 82]. Reasonably, amphiphilic CA derivatives (Figure 2) have also demonstrated significant potentials in gene delivery and therapy. Up to now, popular hydrophilic parts of amphiphilic CAs for interacting with DNA contain amino, guanidinium and tetraalkyl ammonium groups.
Amino-modified amphiphilic CAs
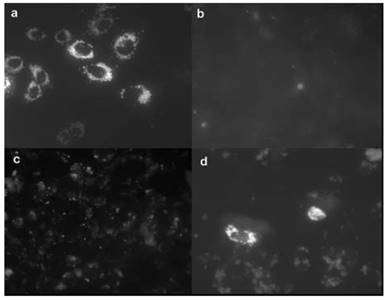
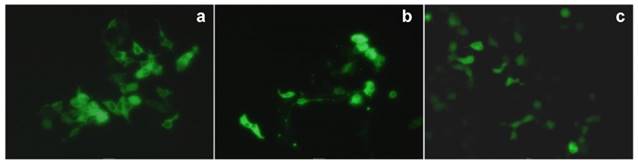
In 2007, Matthews et al. reported amino-modified multicalixarenes (61-63) [83]. Compounds 61-63 can effectively bind to and condense DNA, as revealed by gel electrophoresis. Low cytotoxicity was observed in various cell lines, including Chinese Hamster Ovary (CHO), Human Embryonic Kidney (HEK293) and Human monocytic cell line (THP-1). Among these CAs, only 62, which has aliphatic amines, showed efficient gene transfection in vitro (Figure 3), likely attributed to its relatively high charge-density and its ability to release DNA through endosomal pathway.
In 2015, Sakurai et al. reported amphiphilic calix[4]arenes 64-68 with four amino groups at the upper rim and different lengths of alkyl chains at the lower rim. Highest transfection efficiency was observed with 65 that bears a medium-length alkyl chain with six carbons [84]. Furthermore, in the presence of phosphatidylserine micelles, DNA can be released from the co-assemblies of 65 and plasmid DNA, at pH 5 but not at pH 7. This indicated that the co-assemblies may interact with the late endosomal membrane to release DNA selectively.
In 2018, Candiani et al. developed aminoglycosides modified amphiphilic calix[4]arenes 69-71 [85], which complexed with DNA with a higher affinity than PEI. The nanoassemblies of DNA and these amphiphilic calix[4]arenes showed excellent transfection efficiency and low-to-negligible cytotoxicity in both Hela and U87-MG cells. Moreover, the assemblies also exhibited good antimicrobial activity against Gram-negative bacteria, likely attributed to the antibiotic nature of the aminoglycosides.
Guanidinium-modified amphiphilic CAs
Guanidinium groups possess not only positive charges, but also the geometric complementarity of guanidine N-C-N triad to phosphate O-P-O triad groups of DNA. Ungaro et al. reported water-soluble guanidinium-functionalized CA derivatives (72-81) [86, 87]. Compared with cone-shaped guanidinium calix[4]arene derivatives, conformationally mobile calix[6]arene and calix[8]arene methoxy derivatives failed to promote gene transfection in vitro (Figure 4). Furthermore, calix[4]arenes in a 1,3-alternate conformation showed an intermediate behavior in condensing DNA, revealing the importance of molecular geometry in forming compact particles with DNA. Therefore, the capability of macrocyclic guanidinium CAs in condensing DNA and facilitating gene transfection is highly dependent on their size, lipophilicity, and conformational properties.
Molecular structures of amphiphilic CAs engaged in gene delivery.
Transfection of a plasmid expressing a fluorescent protein that accumulated in mitochondria (pDs2-mito) in CHO cells. (a) Positive control (cells transfected with commercial FuGene1); (b) negative control (PBS buffer); (c) transfection with 63; (d) transfection with 62. Reprinted with permission from ref [83]. Copyright 2007 from Royal Society of Chemistry.
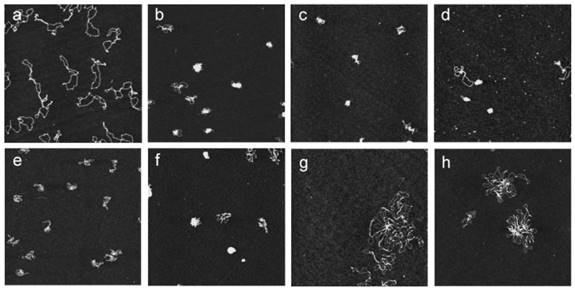
Atomic force microscopy images showing the DNA condensation induced by guanidinium CAs. All images were obtained with supercoiled pEGFPC1 plasmid deposited onto mica at a concentration of 1 nM and with the microscope operating in the tapping mode in air. (a) Plasmid with no CAs added. Plasmid incubated with (b) 1 μM 72; (c) 1 μM 73; (d) 1 μM 74; (e) 1 μM 76; (f) 10 μM 79; (g) 1 μM 77; and (h) 1 μM 78. Reprinted with permission from ref [87]. Copyright 2006 from American Chemical Society.
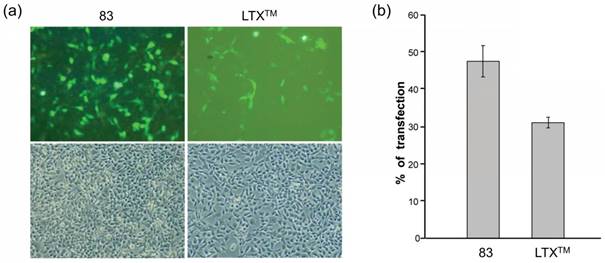
In 2008, they further developed amphiphilic CAs 82-84 and formulated them with DOPE [88]. Attaching guanidinium groups at the lower rim of calix[4]arenes significantly enhanced its gene delivery and transfection efficiency with reduced cytotoxicity, compared to the same macrocycles with the charged groups directly linked at the upper rim. After formulating with DOPE, the co-assembly of 83/DNA behaved even better than that of LTX, a widely used commercial lipofectamine for gene transfection, in RD-4 human Rhabdomyosarcoma (Figure 5) [89]. Furthermore, in 2013, they synthesized positively charged amphiphilic calix[4]arenes 85 and 86 [90], which exhibited high efficiency in DNA delivery and transfection in a variety of cell lines.
Tetraalkylammonium-modified amphiphilic CAs
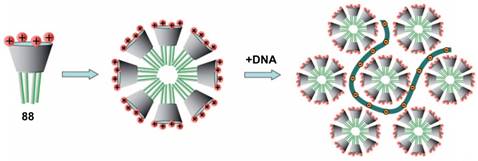
In 2011, Klymchenko et al. reported cationic choline modified calix[4]arenes 87 and 88 [91]. In water, 88 formed micelles with 6 nm diameter at low critical micellar concentration (48 μM), and condensed DNA into small NPs of about 50 nm diameter (Figure 6). In contrast, 87 could not self-assemble into micelles at low concentrations and instead formed large polydispersed complexes with DNA. Consequently, NPs of 88/DNA exhibited much better gene transfection efficiency in cells than the large 87/DNA complexes, indicating that gene delivery of CA/DNA complexes depends strongly on their amphiphilicity.
In 2015, Junquera et al. reported lipoplexes formed by amphiphilic calix[4]arene 89, the DNA pEGFP-C3 (encoding green fluorescent protein), and DOPE [92]. The best transfection conditions of the lipoplexes were that CA molar fraction (n(CA)/(n(CA)+n(DOPE))) is 0.2 and effective charge ratio between the CA and DNA charges, was 20 [92] (Figure 7). The 89-based polycationic lipidic vector can compact and transfect the pEGFP-C3 plasmid at the concentrations with low cytotoxicity.
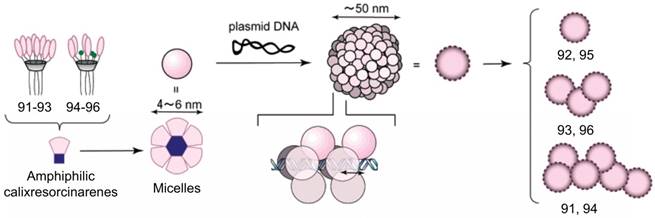
Amphiphilic calixresorcinarenes
Calixresorcinarenes are calixarene analogues based on the condensation of resorcinol and aldehyde. Aoyama et al. reported a number of amphiphilic calix[4]resorcinarenes 90-96 [93-95], decorated with eight (91-93) or five (94-96) disaccharide residues: maltose, lactose or cellobiose, which can form multiple hydrogen bonds with DNA. The calixresorcinarenes formed micelles with dimeters of 5-6 nm. In the presence of DNA, the micelles transformed into compactly packed, well charge-shielded NPs with diameters about 50 nm. Furthermore, the NPs exhibited saccharide-dependent self-aggregation (Figure 8). Hela and HepG2 cells were transfected by these NPs via a nonspecific but highly size-regulated endocytic pathway, where only monomeric NPs possessed substantial transfection activities.
Amphiphilic PAs for gene delivery
PA is a relatively new family of tubular-shaped macrocycle developed by Ogoshi et al. in 2008 [96] and show many applications in various area [34, 97, 98]. The repeating units of PAs are connected through methylene bridges at the para-positions, forming a rigid pillar shape. PAs with n repeating units are often referred to as pillar[n]arenes and the most popular PAs are those with n = 5 and 6. Due to their high symmetry, PAs are a group of fascinating candidates to prepare bola-type amphiphiles. Such bola-type macrocyclic amphiphiles have exhibited some potential in gene delivery.
Transfection experiments performed with 1 nM pEGFP-C1 plasmid, 83/DOPE (10/20 μM) formulation, and lipofectamine LTX to Rhabdomyosarcoma cells. (a) Fluorescence microscopy images (upper row) of the transfected cells as visualized thanks to the expression of the enhanced green fluorescent protein and phase contrast images (lower row) of the corresponding experiments. (b) In vitro transfection efficiency as percentage of transfected cells upon treatment with 83/DOPE formulations and lipofectamine LTX. Reprinted with permission from ref [89]. Copyright 2012 from American Chemical Society.
Simplified scheme of self-assembly of amphiphilic CA 88 into micelles and further formation of the DNA complex. Reprinted with permission from ref [91]. Copyright 2011 from Wiley-VCH Verlag GmbH & Co. KGaA.
Representative images of fluorescence microscopy showing green fluorescent protein expression in HEK293T cells transfected in the presence of serum (+FBS) with TMAC4/DOPE-pDNA lipoplexes at CA molar fractions of 0.2 for effective charge ratio are (a) 20, (b) 40, and (c) control. Reprinted with permission from ref [92]. Copyright 2015 from American Chemical Society.
Hierarchical growth of 91-96 through micelles to NPs upon complexation with DNA. Reprinted with permission from ref [94]. Copyright 2003 from American Chemical Society.
Molecular structures of amphiphilic PAs used for gene delivery.
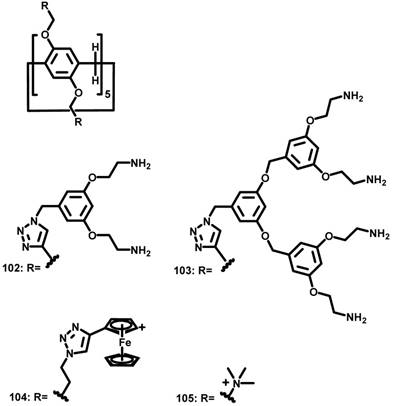
In 2013, Nierengarten et al. reported ammonium-modified pillar[5]arenes 102 and 103 (Figure 9), which have 20 and 40 peripheral ammonium groups, respectively [99]. At a concentration higher than 3 nM, the bola-amphiphilic PA derivatives formed aggregates in an aqueous solution. Gel electrophoresis, ethidium bromide fluorescence and transmission electron microscopy showed that 102 and 103 interacted with and efficiently condensed plasmid DNA (pCMV-Luc) into stable and positively charged polyplexes. The transfection efficiencies of these polyplexes evaluated with Hela cells were moderately lower than that obtained for PEI based polyplexes.
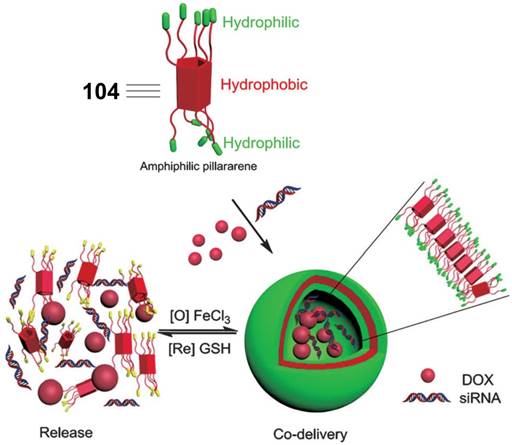
In 2014, Pei et al. reported the glutathione-responsive drug and siRNA co-delivery system based on vesicles formed upon amphiphilic ferrocenium modified pillar[5]arene 104 (Figure 9) [100]. At a concentration higher than 58 μM, 104 formed monolayer-packed vesicle with a diameter about 90 nm. The vesicle is redox-responsive because ferrocenium cations could be reduced to ferrocenyl groups with the molecular polarity altered (Figure 10). The vesicle promoted the cellular uptake of siRNA, and exhibited similar transfection performance with a typical siRNA transfection agent, Lipfectamino 2000. Co-delivery drug resistance gene silencing siRNA (MRP1 siRNA) and doxorubicin re-sensitized and synergistically inhibited SKOV-3 cell with DOX resistance.
In 2017, Junquera et al. reported the co-assembly of polycationic pillar[5]arene 105 (Figure 9) and lipid for gene delivery [101]. Usually, cationic lipids transfer genes well but exhibit moderate to high toxicity, whereas anionic lipids are more biocompatible but do not bind with DNA because of electrostatic repulsion. Divalent cations, such as Ca2+, Mg2+, Mn2+, Co2+, Cd2+, and Zn2+, have been used as “bridge” between anionic lipids and DNA. In this work, the authors found that polycationic pillar[5]arene/DNA exhibited excellent biocompatibility and much more efficient transfection than divalent cations bridged lipids/DNA for this purpose.
Summary and Perspectives
Macrocyclic amphiphiles are considered as a new class of promising vectors for gene delivery on account of their intrinsic features including molecular recognition and self-assembly. In this review, we summarized recently reported works of gene delivery using amphiphilic macrocycles, particularly those bearing cationic charges, including amphiphilic CDs, CAs and PAs (Table 1). Due to their pre-organized framework, multivalent nature and robust amphiphilic aggregation, macrocyclic amphiphiles have stronger interactions with gene than traditional amphiphilic gene vectors, such as liposomes that have suffered from premature gene leakage and thereafter low transfection efficiency. Therefore, macrocyclic amphiphiles-based vectors are often endowed with excellent abilities in gene condensation, delivery and transfection. Moreover, various gene vectors of macrocyclic amphiphiles shown low toxicity (Table 1). When compared with polymeric vectors, macrocyclic amphiphiles have more defined molecular structures with precise size and molecular weights, therefore batch-to-batch consistency, as critical parameters for clinical translation and regulatory approval, would be ensured from precision manufacture and quality control, which are otherwise challenging for polymeric species. Meanwhile, the macrocyclic amphiphiles still maintain the advantages of polymeric vectors, such as robust amphiphilic aggregation and strong binding with gene.
Illustration of the formation of cationic vesicles of 104, and redox-responsive drug/siRNA release. Reprinted with permission from ref [100]. Copyright 2014 from Wiley-VCH Verlag GmbH & Co. KGaA.
As shown in Table 1, among three families of macrocyclic amphiphiles, amphiphilic CDs are the most extensively studied in gene delivery, mainly owing to their good biocompatibility (remarkably, some CD derivatives have been approved for clinical applications). Attributed to facile functionalization of CAs, a variety of amphiphilic CAs have been obtained, thus a large pool of amphiphilic CAs have been made available for possible further studies and screening. Differing from CD and CA, PA is equatorially symmetric, where both rims are identical. As a result, the synthesis of asymmetric amphiphilic PAs is relatively difficult. However, the symmetrical skeleton makes PA quite suitable for building bola-type amphiphiles. As a young member of the macrocyclic families, amphiphilic PAs' applications in gene delivery is still in its infancy. It is also worthy to mention that more and more novel macrocycles have been recently developed [102-110]. Consequently, the emergence of various new macrocyclic (supra-)amphiphiles [111-116] may become valuable new members in the molecular toolbox of gene vectors. Therefore, the research of gene delivery based on macrocyclic amphiphiles will likely make more significant impact and potentially have a bright future in biomedicine.
Key properties of different macrocyclic amphiphiles for gene delivery.
| CD | CA | PA | |
|---|---|---|---|
| Parent structures of the scaffold | 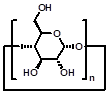 | 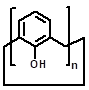 | 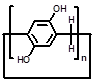 |
| Symmetry | Asymmetric | Asymmetric | Symmetric |
| Types of amphiphiles | Rim-differentiated macrocyclic amphiphiles | Rim-differentiated macrocyclic amphiphiles | Bola type macrocyclic amphiphiles |
| Biocompatibility | Good biocompatibility; some CD/drug complexes were marketed [4, 117]; modification related toxicity need further studied | Some amphiphilic calixarenes have negligible toxicity [80, 90]; long-term toxicity need further studied | Some amphiphilic pillararenes have negligible toxicity [99]; long-term toxicity need further studied |
| Compound numbers summarized in this review | 60 | 41 | 4 |
It can be envisaged that there are still some challenges for further development of macrocyclic amphiphiles as novel gene vectors, particularly their practical applications in clinical practice. First, very few studies examined the use of amphiphilic macrocycles for gene delivery and transfection in vivo to fully understand the biocompatibility, distribution and pharmacokinetics of the systems in a pre-clinical model. Second, although the guest-binding properties of macrocyclic amphiphiles have been studied in a few cases, the full potential of guest recognition was not fully leveraged yet in their applications as gene vectors. One can design smart gene delivery systems in virtue of selective host-guest recognition of overexpressed biomarkers [50, 118-120]. This direction of research is very attractive but full of challenges because systemic screening of selective recognition of specific biomarkers by macrocyclic receptors would be needed. And such selectivity should not be compromised by the complex physiological environments. Third, some crucial questions for clinical application of macrocyclic amphiphiles, such as their long-term toxicity, metabolisms and biological fates, and whether the benefits of using them would outweigh the associated risks, still need to be systemically addressed, at least with clinically relevant animal models. Nevertheless, these challenges may be turned into opportunities for researchers to pinpoint key areas for future research and development. In addition to designing and developing new research kits (amphiphilic macrocycles) and continuing to test their gene delivery capabilities, interdisciplinary approaches involving scientists in chemistry, biology and materials sciences, as well as clinicians, would be required to further advance these fancy toys from benchtop to bedside.
Abbreviations
CA: calixarene; CD: cyclodextrin; DOPE: dioleoylphosphatidylethanolamine; FA: folic acid; NPs: nanoparticles; PA: pillararene; PEI: polyethyleneimine; siRNA: small interfering RNA.
Acknowledgements
This work was supported by NSFC (51873090, 21672112 and 21871301), the Fundamental Research Funds for the Central Universities, Program of Tianjin Young Talents, Science and Technology Development Fund, Macao SAR (FDCT 030/2017/A1 and FDCT 0121/2018/A3), which are gratefully acknowledged. In addition, Ms. Qiaoxian Huang and Dr. Dong-Sheng Guo are financially supported by UM Macao PhD Scholarship and UM Macao Distinguished Visiting Scholar Scheme, respectively.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Gogarten JP, Lawrence JG, Doolittle WF. Prokaryotic Evolution in Light of Gene Transfer. Mol Biol Evol. 2002;19:2226-38
2. Keeling PJ, Palmer JD. Horizontal gene transfer in eukaryotic evolution. Nat Rev Genet. 2008;9:605-18
3. Jiménez Blanco JL, Benito JM, Ortiz Mellet C, García Fernández JM. Molecular nanoparticle-based gene delivery systems. J Drug Deliv Sci Technol. 2017;42:18-37
4. Mellet CO, Fernández JMG, Benito JM. Cyclodextrin-based gene delivery systems. Chem Soc Rev. 2011;40:1586-608
5. Jin L, Zeng X, Liu M, Deng Y, He N. Current progress in gene delivery technology based on chemical methods and nano-carriers. Theranostics. 2014;4:240-55
6. Dominska M, Dykxhoorn DM. Breaking down the barriers: siRNA delivery and endosome escape. J Cell Sci. 2010;123:1183
7. Nishikawa M, Huang L. Nonviral Vectors in the New Millennium: Delivery Barriers in Gene Transfer. Hum Gene Ther. 2001;12:861-70
8. Whitehead KA, Langer R, Anderson DG. Knocking down barriers: advances in siRNA delivery. Nat Rev Drug Discov. 2009;8:129-38
9. Paul S, Regulier E, Rooke R, Stoeckel F, Geist M, Homann H. et al. Tumor gene therapy by MVA-mediated expression of T-cell-stimulating antibodies. Cancer Gene Ther. 2002;9:470-7
10. Odin L, Favrot M, Poujol D, Michot JP, Moingeon P, Tartaglia J. et al. Canarypox virus expressing wild type p53 for gene therapy in murine tumors mutated in p53. Cancer Gene Ther. 2001;8:87-98
11. Lin Q, Chen J, Zhang Z, Zheng G. Lipid-based nanoparticles in the systemic delivery of siRNA. Nanomedicine. 2013;9:105-20
12. Yang J, Zhang Q, Chang H, Cheng Y. Surface-engineered dendrimers in gene delivery. Chem Rev. 2015;115:5274-300
13. Liu H, Wang H, Yang W, Cheng Y. Disulfide cross-linked low generation dendrimers with high gene transfection efficacy, low cytotoxicity, and low cost. J Am Chem Soc. 2012;134:17680-7
14. Ghosh P, Han G, De M, Kim CK, Rotello VM. Gold nanoparticles in delivery applications. Adv Drug Deliv Rev. 2008;60:1307-15
15. Liu J, Stace-Naughton A, Brinker CJ. Silica nanoparticle supported lipid bilayers for gene delivery. Chem Commun. 2009;34:5100-2
16. Ping Y, Liu C-D, Tang G-P, Li J-S, Li J, Yang W-T. et al. Functionalization of Chitosan via Atom Transfer Radical Polymerization for Gene Delivery. Adv Funct Mater. 2010;20:3106-16
17. Xu FJ, Yang WT. Polymer vectors via controlled/living radical polymerization for gene delivery. Prog Polym Sci. 2011;36:1099-131
18. Liu G-Y, Chen C-J, Ji J. Biocompatible and biodegradable polymersomes as delivery vehicles in biomedical applications. Soft matter. 2012;8:8811-21
19. Dahlman JE, Barnes C, Khan OF, Thiriot A, Jhunjunwala S, Shaw TE. et al. In vivo endothelial siRNA delivery using polymeric nanoparticles with low molecular weight. Nat Nanotechnol. 2014;9:648-55
20. Gabrielson NP, Cheng J. Multiplexed supramolecular self-assembly for non-viral gene delivery. Biomaterials. 2010;31:9117-27
21. Yang Y, Liu F, Liu X, Xing B. NIR light controlled photorelease of siRNA and its targeted intracellular delivery based on upconversion nanoparticles. Nanoscale. 2013;5:231-8
22. Wang Y, Gao SJ, Ye WH, Yoon HS, Yang YY. Co-delivery of drugs and DNA from cationic core-shell nanoparticles self-assembled from a biodegradable copolymer. Nat Mater. 2006;5:791-6
23. Zheng B, Wang F, Dong S, Huang F. Supramolecular polymers constructed by crown ether-based molecular recognition. Chem Soc Rev. 2012;41:1621-36
24. Ravoo BJ, Darcy R. Cyclodextrin Bilayer Vesicles. Angew Chem Int Ed. 2000;39:4324-6
25. Chen Y, Liu Y. Cyclodextrin-based bioactive supramolecular assemblies. Chem Soc Rev. 2010;39:495-505
26. Jia L, Xu J-P, Li D, Pang S-P, Fang Y, Song Z-G. et al. Fluorescence detection of alkaline phosphatase activity with β-cyclodextrin-modified quantum dots. Chem Commun. 2010;46:7166-8
27. Liu Y, Zhao D, Ma R, Xiong Da, An Y, Shi L. Chaperone-like α-cyclodextrins assisted self-assembly of double hydrophilic block copolymers in aqueous medium. Polymer. 2009;50:855-9
28. Guo D-S, Liu Y. Supramolecular chemistry of p-sulfonatocalix[n]arenes and its biological applications. Acc Chem Res. 2014;47:1925-34
29. Zheng Z, Geng W-C, Gao J, Wang Y-Y, Sun H, Guo D-S. Ultrasensitive and specific fluorescence detection of a cancer biomarker via nanomolar binding to a guanidinium-modified calixarene. Chem Sci. 2018;9:2087-91
30. Guo D-S, Wang K, Wang Y-X, Liu Y. Cholinesterase-Responsive Supramolecular Vesicle. J Am Chem Soc. 2012;134:10244-50
31. Lee JW, Samal S, Selvapalam N, Kim H-J, Kim K. Cucurbituril Homologues and Derivatives: New Opportunities in Supramolecular Chemistry. Acc Chem Res. 2003;36:621-30
32. Barrow SJ, Kasera S, Rowland MJ, del Barrio J, Scherman OA. Cucurbituril-Based Molecular Recognition. Chem Rev. 2015;115:12320-406
33. Xie J, Peng HJ, Huang JQ, Xu WT, Chen X, Zhang Q. A Supramolecular Capsule for Reversible Polysulfide Storage/Delivery in Lithium-Sulfur Batteries. Angew Chem Int Ed. 2017;56:16223-7
34. Xue M, Yang Y, Chi X, Zhang Z, Huang F. Pillararenes, A New Class of Macrocycles for Supramolecular Chemistry. Acc Chem Res. 2012;45:1294-308
35. Li C. Pillararene-based supramolecular polymers: from molecular recognition to polymeric aggregates. Chem Commun. 2014;50:12420-33
36. Yang K, Pei Y, Wen J, Pei Z. Recent advances in pillar[n]arenes: synthesis and applications based on host-guest interactions. Chem Commun. 2016;52:9316-26
37. Feng W, Jin M, Yang K, Pei Y, Pei Z. Supramolecular delivery systems based on pillararenes. Chem Commun. 2018;54:13626-40
38. Liu Z, Nalluri SKM, Stoddart JF. Surveying macrocyclic chemistry: from flexible crown ethers to rigid cyclophanes. Chem Soc Rev. 2017;46:2459-78
39. Xie J, Bogliotti N. Synthesis and Applications of Carbohydrate-Derived Macrocyclic Compounds. Chem Rev. 2014;114:7678-739
40. Jie K, Zhou Y, Yao Y, Huang F. Macrocyclic amphiphiles. Chem Soc Rev. 2015;44:3568-87
41. Shinkai S, Mori S, Koreishi H, Tsubaki T, Manabe O. Hexasulfonated calix[6]arene derivatives: a new class of catalysts, surfactants, and host molecules. J Am Chem Soc. 1986;108:2409-16
42. Zadmard R, Arendt M, Schrader T. Multipoint Recognition of Basic Proteins at a Membrane Model. J Am Chem Soc. 2004;126:7752-3
43. Pan Y-C, Tian H-W, Peng S, Hu X-Y, Guo D-S. Molecular recognition of sulfonatocalixarene with organic cations at the self-assembled interface: a thermodynamic investigation. Chin Chem Lett. 2017;28:787-92
44. Zheng Z, Geng W-C, Gao J, Mu Y-J, Guo D-S. Differential calixarene receptors create patterns that discriminate glycosaminoglycans. Org Chem Front. 2018;5:2685-91
45. Zhang H, Ma X, Nguyen KT, Zhao Y. Biocompatible Pillararene-Assembly-Based Carriers for Dual Bioimaging. ACS nano. 2013;7:7853-63
46. Xu Z, Peng S, Wang Y-Y, Zhang J-K, Lazar AI, Guo D-S. Broad-Spectrum Tunable Photoluminescent Nanomaterials Constructed from a Modular Light-Harvesting Platform Based on Macrocyclic Amphiphiles. Adv Mater. 2016;28:7666-71
47. Martin AD, Boulos RA, Stubbs KA, Raston CL. Phosphonated calix[4]arene-based amphiphiles as scaffolds for fluorescent nano-fibres. Chem Commun. 2011;47:7329-31
48. Geng W-C, Liu Y-C, Wang Y-Y, Xu Z, Zheng Z, Yang C-B. et al. A self-assembled white-light-emitting system in aqueous medium based on a macrocyclic amphiphile. Chem Commun. 2017;53:392-5
49. Xu Z, Gonzalez-Abradelo D, Li J, Strassert CA, Ravoo BJ, Guo D-S. Supramolecular color-tunable photoluminescent materials based on a chromophore cascade as security inks with dual encryption. Mater Chem Front. 2017;1:1847-52
50. Gao J, Li J, Geng W-C, Chen F-Y, Duan X, Zheng Z. et al. Biomarker Displacement Activation: A General Host-Guest Strategy for Targeted Phototheranostics in Vivo. J Am Chem Soc. 2018;140:4945-53
51. Szejtli J. Introduction and General Overview of Cyclodextrin Chemistry. Chem Rev. 1998;98:1743-54
52. Arima H, Hayashi Y, Higashi T, Motoyama K. Recent advances in cyclodextrin delivery techniques. Expert Opin Drug Deliv. 2015;12:1425-41
53. Cryan SA, Donohue R, Ravoo BJ, Darcy R, O'Driscoll CM. Cationic cyclodextrin amphiphiles as gene delivery vectors. J Drug Deliv Sci Technol. 2004;14:57-62
54. Godinho BM, Ogier JR, Darcy R, O'Driscoll CM, Cryan JF. Self-assembling modified beta-cyclodextrin nanoparticles as neuronal siRNA delivery vectors: focus on Huntington's disease. Mol Pharmacol. 2013;10:640-9
55. O'Mahony AM, Ogier J, Desgranges S, Cryan JF, Darcy R, O'Driscoll CM. A click chemistry route to 2-functionalised PEGylated and cationic beta-cyclodextrins: co-formulation opportunities for siRNA delivery. Org Biomol Chem. 2012;10:4954-60
56. Guo J, Russell EG, Darcy R, Cotter TG, McKenna SL, Cahill MR. et al. Antibody-Targeted Cyclodextrin-Based Nanoparticles for siRNA Delivery in the Treatment of Acute Myeloid Leukemia: Physicochemical Characteristics, in Vitro Mechanistic Studies, and ex Vivo Patient Derived Therapeutic Efficacy. Mol Pharmacol. 2017;14:940-52
57. Byrne C, Sallas F, Rai DK, Ogier J, Darcy R. Poly-6-cationic amphiphilic cyclodextrins designed for gene delivery. Org Biomol Chem. 2009;7:3763-71
58. MJ ON, Guo J, Byrne C, Darcy R, CM OD. Mechanistic studies on the uptake and intracellular trafficking of novel cyclodextrin transfection complexes by intestinal epithelial cells. Int J Pharm. 2011;413:174-83
59. O'Neill MJ, O'Mahony AM, Byrne C, Darcy R, O'Driscoll CM. Gastrointestinal gene delivery by cyclodextrins-in vitro quantification of extracellular barriers. Int J Pharm. 2013;456:390-9
60. Ortega-Caballero F, Mellet CO, Le Gourrierec L, Guilloteau N, Di Giorgio C, Vierling P. et al. Tailoring beta-cyclodextrin for DNA complexation and delivery by homogeneous functionalization at the secondary face. Org Lett. 2008;10:5143-6
61. Mendez-Ardoy A, Guilloteau N, Di Giorgio C, Vierling P, Santoyo-Gonzalez F, Ortiz Mellet C. et al. beta-Cyclodextrin-based polycationic amphiphilic "click" clusters: effect of structural modifications in their DNA complexing and delivery properties. J Org Chem. 2011;76:5882-94
62. Gallego-Yerga L, Lomazzi M, Franceschi V, Sansone F, Ortiz Mellet C, Donofrio G. et al. Cyclodextrin- and calixarene-based polycationic amphiphiles as gene delivery systems: a structure-activity relationship study. Org Biomol Chem. 2015;13:1708-23
63. Diaz-Moscoso A, Vercauteren D, Rejman J, Benito JM, Ortiz Mellet C, De Smedt SC. et al. Insights in cellular uptake mechanisms of pDNA-polycationic amphiphilic cyclodextrin nanoparticles (CDplexes). J Control Release. 2010;143:318-25
64. Mendez-Ardoy A, Urbiola K, Aranda C, Ortiz-Mellet C, Garcia-Fernandez JM, de Ilarduya CT. Polycationic amphiphilic cyclodextrin-based nanoparticles for therapeutic gene delivery. Nanomedicine (Lond). 2011;6:1697-707
65. Martinez A, Bienvenu C, Jimenez Blanco JL, Vierling P, Mellet CO, Garcia Fernandez JM. et al. Amphiphilic oligoethyleneimine-beta-cyclodextrin "click" clusters for enhanced DNA delivery. J Org Chem. 2013;78:8143-8
66. Mendez-Ardoy A, Gomez-Garcia M, Ortiz Mellet C, Sevillano N, Giron MD, Salto R. et al. Preorganized macromolecular gene delivery systems: amphiphilic beta-cyclodextrin "click clusters". Org Biomol Chem. 2009;7:2681-4
67. Gallego-Yerga L, Gonzalez-Alvarez MJ, Mayordomo N, Santoyo-Gonzalez F, Benito JM, Ortiz Mellet C. et al. Dynamic self-assembly of polycationic clusters based on cyclodextrins for pH-sensitive DNA nanocondensation and delivery by component design. Chem Eur J. 2014;20:6622-7
68. Remaut K, De Clercq E, Andries O, Rombouts K, Van Gils M, Cicchelero L. et al. Aerosolized Non-viral Nucleic Acid Delivery in the Vaginal Tract of Pigs. Pharm Res. 2016;33:384-94
69. Minnaert A-K, Dakwar GR, Benito JM, García Fernández JM, Ceelen W, De Smedt SC. et al. High-Pressure Nebulization as Application Route for the Peritoneal Administration of siRNA Complexes. Macromolecular bioscience. 2017;17:1700024
70. Martinez-Negro M, Caracciolo G, Palchetti S, Pozzi D, Capriotti AL, Cavaliere C. et al. Biophysics and protein corona analysis of Janus cyclodextrin-DNA nanocomplexes. Efficient cellular transfection on cancer cells. Biochim Biophys Acta Gen Subj. 2017;1861:1737-49
71. Manzanares D, Araya-Durán I, Gallego-Yerga L, Játiva P, Márquez-Miranda V, Canan J. et al. Molecular determinants for cyclo-oligosaccharide-based nanoparticle-mediated effective siRNA transfection. Nanomedicine. 2017;12:1607-21
72. Aranda C, Urbiola K, Mendez Ardoy A, Garcia Fernandez JM, Ortiz Mellet C, de Ilarduya CT. Targeted gene delivery by new folate-polycationic amphiphilic cyclodextrin-DNA nanocomplexes in vitro and in vivo. Eur J Pharm Biopharm. 2013;85:390-7
73. Diaz-Moscoso A, Guilloteau N, Bienvenu C, Mendez-Ardoy A, Blanco JL, Benito JM. et al. Mannosyl-coated nanocomplexes from amphiphilic cyclodextrins and pDNA for site-specific gene delivery. Biomaterials. 2011;32:7263-73
74. Symens N, Mendez-Ardoy A, Diaz-Moscoso A, Sanchez-Fernandez E, Remaut K, Demeester J. et al. Efficient transfection of hepatocytes mediated by mRNA complexed to galactosylated cyclodextrins. Bioconjug Chem. 2012;23:1276-89
75. Aguilar Moncayo EM, Guilloteau N, Bienvenu C, Jiménez Blanco JL, Di Giorgio C, Vierling P. et al. Cyclodextrin-scaffolded amphiphilic aminoglucoside clusters: self-assembling and gene delivery capabilities. New J Chem. 2014;38:5215-25
76. McMahon A, O'Neill MJ, Gomez E, Donohue R, Forde D, Darcy R. et al. Targeted gene delivery to hepatocytes with galactosylated amphiphilic cyclodextrins. J Pharm Pharmacol. 2012;64:1063-73
77. Guo D-S, Liu Y. Calixarene-based supramolecular polymerization in solution. Chem Soc Rev. 2012;41:5907-21
78. Volker B. Calixarenes, Macrocycles with (Almost) Unlimited Possibilities. Angew Chem Int Ed. 1995;34:713-45
79. Geng W-C, Jia S, Zheng Z, Li Z, Ding D, Guo D-S. A Noncovalent Fluorescence Turn-on Strategy for Hypoxia Imaging. Angew Chem Int Ed. 2019 doi:10.1002/anie.201813397
80. Rodik RV, Klymchenko AS, Mely Y, Kalchenko VI. Calixarenes and related macrocycles as gene delivery vehicles. J Incl Phenom Macrocycl Chem. 2014;80:189-200
81. Helttunen K, Shahgaldian P. Self-assembly of amphiphilic calixarenes and resorcinarenes in water. New J Chem. 2010;34:2704-14
82. Basilio N, Francisco V, Garcia-Rio L. Aggregation of p-Sulfonatocalixarene-Based Amphiphiles and Supra-Amphiphiles. Int J Mol Sci. 2013;14:3140-57
83. Lalor R, DiGesso JL, Mueller A, Matthews SE. Efficient gene transfection with functionalised multicalixarenes. Chem Commun. 2007;46:4907-9
84. Mochizuki S, Nishina K, Fujii S, Sakurai K. The transfection efficiency of calix[4]arene-based lipids: the role of the alkyl chain length. Biomater Sci. 2015;3:317-22
85. Bono N, Pennetta C, Sganappa A, Giupponi E, Sansone F, Volonterio A. et al. Design and synthesis of biologically active cationic amphiphiles built on the calix[4]arene scaffold. Int J Pharm. 2018;549:436-45
86. Dudic M, Colombo A, Sansone F, Casnati A, Donofrio G, Ungaro R. A general synthesis of water soluble upper rim calix[n]arene guanidinium derivatives which bind to plasmid DNA. Tetrahedron. 2004;60:11613-8
87. Sansone F, Dudic M, Donofrio G, Rivetti C, Baldini L, Casnati A. et al. DNA condensation and cell transfection properties of guanidinium calixarenes: dependence on macrocycle lipophilicity, size, and conformation. J Am Chem Soc. 2006;128:14528-36
88. Bagnacani V, Sansone F, Donofrio G, Baldini L, Casnati A, Ungaro R. Macrocyclic nonviral vectors: high cell transfection efficiency and low toxicity in a lower rim guanidinium calix[4]arene. Org Lett. 2008;10:3953-6
89. Bagnacani V, Franceschi V, Fantuzzi L, Casnati A, Donofrio G, Sansone F. et al. Lower rim guanidinocalix[4]arenes: macrocyclic nonviral vectors for cell transfection. Bioconjug Chem. 2012;23:993-1002
90. Bagnacani V, Franceschi V, Bassi M, Lomazzi M, Donofrio G, Sansone F. et al. Arginine clustering on calix[4]arene macrocycles for improved cell penetration and DNA delivery. Nat Commun. 2013;4:1721
91. Rodik RV, Klymchenko AS, Jain N, Miroshnichenko SI, Richert L, Kalchenko VI. et al. Virus-sized DNA nanoparticles for gene delivery based on micelles of cationic calixarenes. Chem Eur J. 2011;17:5526-38
92. Barran-Berdon AL, Yelamos B, Garcia-Rio L, Domenech O, Aicart E, Junquera E. Polycationic Macrocyclic Scaffolds as Potential Non-Viral Vectors of DNA: A Multidisciplinary Study. ACS Appl Mater Interfaces. 2015;7:14404-14
93. Aoyama Y, Kanamori T, Nakai T, Sasaki T, Horiuchi S, Sando S. et al. Artificial viruses and their application to gene delivery. Size-controlled gene coating with glycocluster nanoparticles. J Am Chem Soc. 2003;125:3455-7
94. Nakai T, Kanamori T, Sando S, Aoyama Y. Remarkably size-regulated cell invasion by artificial viruses. Saccharide-dependent self-aggregation of glycoviruses and its consequences in glycoviral gene delivery. J Am Chem Soc. 2003;125:8465-75
95. Horiuchi S, Aoyama Y. Systematic lactose-functionalization of amphiphilic octaamine macrocycle as a gene carrier. Optimization of the charge, size, toxicity, and receptor factors for hepatocyte targeting. J Control Release. 2006;116:107-14
96. Ogoshi T, Kanai S, Fujinami S, Yamagishi T-a, Nakamoto Y. para-Bridged Symmetrical Pillar[5]arenes: Their Lewis Acid Catalyzed Synthesis and Host-Guest Property. J Am Chem Soc. 2008;130:5022-3
97. Wang R, Sun Y, Zhang F, Song M, Tian D, Li H. Temperature-Sensitive Artificial Channels through Pillar[5]arene-based Host-Guest Interactions. Angew Chem Int Ed. 2017;56:5294-8
98. Jie K, Zhou Y, Li E, Zhao R, Huang F. Separation of Aromatics/Cyclic Aliphatics by Nonporous Adaptive Pillararene Crystals. Angew Chem Int Ed. 2018;57:12845-9
99. Nierengarten I, Nothisen M, Sigwalt D, Biellmann T, Holler M, Remy JS. et al. Polycationic pillar[5]arene derivatives: interaction with DNA and biological applications. Chem Eur J. 2013;19:17552-8
100. Chang Y, Yang K, Wei P, Huang S, Pei Y, Zhao W. et al. Cationic vesicles based on amphiphilic pillar[5]arene capped with ferrocenium: a redox-responsive system for drug/siRNA co-delivery. Angew Chem Int Ed. 2014;53:13126-30
101. Barrán-Berdón AL, Martínez-Negro M, García-Río L, Domènech Ò, Tros de Ilarduya C, Aicart E. et al. A biophysical study of gene nanocarriers formed by anionic/zwitterionic mixed lipids and pillar[5]arene polycationic macrocycles. J Mater Chem B. 2017;5:3122-31
102. Wu J-R, Yang Y-W. New opportunities in synthetic macrocyclic arenes. Chem Commun. 2019 doi:10.1039/C8CC09374A
103. Li B, Huang X, Dai L, Cui L, Li J, Jia X. et al. Terphen[n]arenes and Quaterphen[n]arenes (n = 3-6): One-pot Synthesis, Self-assembly into Supramolecular Gels, and Iodine Capture. Angew Chem Int Ed. 2019 doi:10.1002/anie.201813972
104. Huang G-B, Wang S-H, Ke H, Yang L-P, Jiang W. Selective Recognition of Highly Hydrophilic Molecules in Water by Endo-Functionalized Molecular Tubes. J Am Chem Soc. 2016;138:14550-3
105. Yang L-P, Liu W-E, Jiang W. Naphthol-based macrocyclic receptors. Tetrahedron Lett. 2016;57:3978-85
106. Zhang G-W, Li P-F, Meng Z, Wang H-X, Han Y, Chen C-F. Triptycene-Based Chiral Macrocyclic Hosts for Highly Enantioselective Recognition of Chiral Guests Containing a Trimethylamino Group. Angew Chem Int Ed. 2016;55:5304-8
107. Fa S-X, Wang X-D, Wang Q-Q, Ao Y-F, Wang D-X, Wang M-X. Multiresponsive Vesicles Composed of Amphiphilic Azacalix[4]pyridine Derivatives. ACS Appl Mater Interfaces. 2017;9:10378-82
108. Wang M-X. Heterocalixaromatics, new generation macrocyclic host molecules in supramolecular chemistry. Chem Commun. 2008;38:4541-51
109. Wu JR, Mu AU, Li B, Wang CY, Fang L, Yang YW. Desymmetrized Leaning Pillar[6]arene. Angew Chem Int Ed. 2018;57:9853-8
110. Jiménez Blanco JL, Ortega-Caballero F, Blanco-Fernández L, Carmona T, Marcelo G, Martínez-Negro M. et al. Trehalose-based Janus cyclooligosaccharides: the “Click” synthesis and DNA-directed assembly into pH-sensitive transfectious nanoparticles. Chem Commun. 2016;52:10117-20
111. Zhu H, Shangguan L, Shi B, Yu G, Huang F. Recent progress in macrocyclic amphiphiles and macrocyclic host-based supra-amphiphiles. Mater Chem Front. 2018;2:2152-74
112. Wang C, Wang Z, Zhang X. Amphiphilic Building Blocks for Self-Assembly: From Amphiphiles to Supra-amphiphiles. Acc Chem Res. 2012;45:608-18
113. Chi X, Zhang H, Vargas-Zúñiga GI, Peters GM, Sessler JL. A Dual-Responsive Bola-Type Supra-amphiphile Constructed from a Water-Soluble Calix[4]pyrrole and a Tetraphenylethene-Containing Pyridine Bis-N-oxide. J Am Chem Soc. 2016;138:5829-32
114. Yu G, Jie K, Huang F. Supramolecular Amphiphiles Based on Host-Guest Molecular Recognition Motifs. Chem Rev. 2015;115:7240-303
115. Zhang X, Wang C. Supramolecular amphiphiles. Chem Soc Rev. 2011;40:94-101
116. Gallego-Yerga L, Blanco-Fernández L, Urbiola K, Carmona T, Marcelo G, Benito JM. et al. Host-Guest-Mediated DNA Templation of Polycationic Supramolecules for Hierarchical Nanocondensation and the Delivery of Gene Material. Chem Eur J. 2015;21:12093-104
117. Davis ME, Brewster ME. Cyclodextrin-based pharmaceutics: past, present and future. Nat Rev Drug Discovery. 2004;3:1023
118. Hao Q, Chen Y, Huang Z, Xu JF, Sun Z, Zhang X. Supramolecular Chemotherapy: Carboxylated Pillar[6]arene for Decreasing Cytotoxicity of Oxaliplatin to Normal Cells and Improving Its Anticancer Bioactivity Against Colorectal Cancer. ACS Appl Mater Interfaces. 2018;10:5365-72
119. Chen H, Chen Y, Wu H, Xu JF, Sun Z, Zhang X. Supramolecular polymeric chemotherapy based on cucurbit[7]uril-PEG copolymer. Biomaterials. 2018;178:697-705
120. Chen Y, Huang Z, Zhao H, Xu JF, Sun Z, Zhang X. Supramolecular Chemotherapy: Cooperative Enhancement of Antitumor Activity by Combining Controlled Release of Oxaliplatin and Consuming of Spermine by Cucurbit[7]uril. ACS Appl Mater Interfaces. 2017;9:8602-8
Author contact
![]() Corresponding authors: E-mail: dshguoedu.cn; Email: rwangedu.mo
Corresponding authors: E-mail: dshguoedu.cn; Email: rwangedu.mo
 Global reach, higher impact
Global reach, higher impact