13.3
Impact Factor
Theranostics 2019; 9(10):2856-2867. doi:10.7150/thno.33595 This issue Cite
Research Paper
QuatCy: A Heptamethine Cyanine Modification With Improved Characteristics
1. Department of Chemistry, Texas A & M University, Box 30012, College Station, TX 77842, USA
2. Gordon Center for Medical Imaging, Department of Radiology, Massachusetts General Hospital and Harvard Medical School, Boston, MA 02114, USA
Abstract
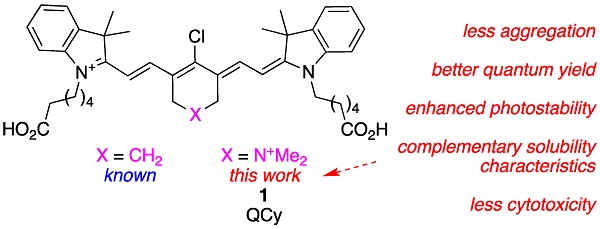
A major restriction on optical imaging techniques is the range of available fluorophores that are compatible with aqueous media without aggregation, absorb light above 750 nm with high extinction coefficients, fluoresce with relatively high quantum yields, and resist photodecomposition. Indocyanine green (ICG or A in this paper) is an important example of a fluorophore that fits this description. Other dyes that are becoming increasingly prevalent are select heptamethine cyanine dyes (Cy7) which feature a cyclohexyl framework to rigidify the conjugated alkenes, and meso-chlorine substitution; MHI-148 (B) is one example.
Methods: Research described here was initiated to uncover the consequences of a simple isoelectronic substitution to MHI-148 that replaces a cyclohexyl methylene with a dialkyl ammonium fragment. Solubility experiments were carried out in aqueous and cell culture media, photophysical properties including fluorescence quantum yields, brightness and stability were measured. Moreover, in vivo pharmacokinetics, distribution and tumor seeking properties were also explored.
Results: Modification to incorporate dialkyl ammonium fragment leads to a brighter, more photostable fluorophore, with a decreased tendency to aggregation, complementary solubility characteristics, and a lower cytotoxicity.
Conclusion: All the above-mentioned parameters are favorable for many anticipated applications of the new dye we now call quaternary cyanine-7 or QuatCy.
Keywords: heptamethine cyanine, fluorescence imaging, tumor seeking, near-infrared
 Global reach, higher impact
Global reach, higher impact