13.3
Impact Factor
Theranostics 2019; 9(8):2361-2379. doi:10.7150/thno.29628 This issue Cite
Research Paper
Induction of nuclear protein-1 by thyroid hormone enhances platelet-derived growth factor A mediated angiogenesis in liver cancer
1. Department of Biochemistry, College of Medicine, Chang-Gung University, Taoyuan, Taiwan
2. Department of Biomedical Sciences, College of Medicine, Chang-Gung University, Taoyuan, Taiwan
3. Division of Pulmonary Medicine, Department of Internal Medicine, Shuang Ho Hospital, Taipei Medical University, New Taipei City, Taiwan
4. Division of Pulmonary Medicine, Department of Internal Medicine, School of Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan
5. Liver Research Center, Chang Gung Memorial Hospital, Linkou, Taoyuan, Taiwan
6. Radiation Biology Research Center, Institute for Radiological Research, Chang Gung University / Chang Gung Memorial Hospital, Linkou, Taoyuan, Taiwan
7. Department of Pathology, Chang Gung Memorial Hospital and Chang Gung University, Taoyuan, Taiwan
8. Research Center for Chinese Herbal Medicine, College of Human Ecology, Chang Gung University of Science and Technology, Taoyuan, Taiwan
Abstract
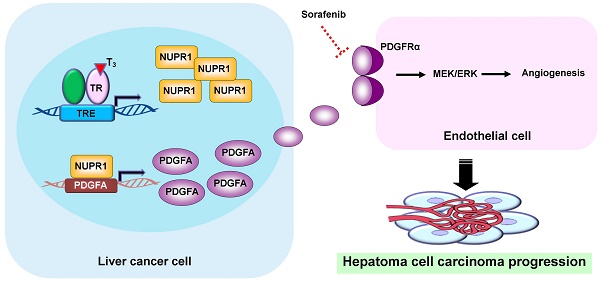
Background & Aims: Hepatocellular carcinoma (HCC) is among the leading causes of cancer deaths worldwide. Many studies indicate that disruption of cellular thyroid hormone signaling promotes HCC progression. However, the mechanisms underlying the regulation of genes downstream of thyroid hormone actions in HCC have remained elusive. In the current study, we identified NUPR1 (nuclear protein-1), a stress-induced protein that overexpresses in various neoplasia, is upregulated by triiodothyronine/thyroid hormone receptor (T3/TR) signaling and aimed to elucidate its role in angiogenesis in cancer progression.
Methods: Quantitative reverse transcription-PCR, luciferase promoter and chromatin immunoprecipitation assays were performed to identify the NUPR1 regulatory mechanism by T3/TR. In vitro and In vivo vascular formations were performed to detect the angiogenic function of NUPR1. Human angiogenesis arrays were performed to identify the downstream angiogenic pathway. The sorafenib resistant ability of TR/NUPR1 was further examined in vitro and in vivo. Clinical relevance of TR, NUPR1 and platelet-derived growth factor A (PDGFA) were investigate in HCC samples using qRT-PCR and western blot.
Results: Our experiments disclosed positive regulation of NUPR1 expression by T3/TR through direct binding to the -2066 to -1910 region of the NUPR1 promoter. Elevated NUPR1 and TR expression link to poor survival in clinical HCC specimens. An analysis of clinicopathological parameters showed that expression of NUPR1 is associated with vascular invasion and pathology stage. Functional studies revealed that NUPR1 induced endothelial cell angiogenesis in vitro and in vivo. Using a human angiogenesis array, we identified PDGFA as a target of NUPR1 in the downstream angiogenic pathway. NUPR1 induced transcription of PDGFA through direct binding to the corresponding promoter region, and inhibition of the PDGFA signaling pathway impaired angiogenesis in human umbilical vein endothelial cells (HUVECs). Notably, the angiogenic effects of NUPR1/PDGFA were mediated by the MEK/ERK signaling pathway. TR/NUPR1 expression increased cell viability and resistance to sorafenib treatment. Moreover NUPR1 expression was positively correlated with TRα, TRβ, and PDGFA expression.
Conclusions: We propose that the T3/TR/NUPR1/PDGFA/MEK/ERK axis has a vital role in hepatocarcinogenesis and suggest NUPR1 as a potential therapeutic target in HCC.
Keywords: Thyroid hormone receptor, NUPR1, Hepatocellular carcinoma, PDGFA, sorafenib
 Global reach, higher impact
Global reach, higher impact