13.3
Impact Factor
Theranostics 2019; 9(8):2315-2324. doi:10.7150/thno.30254 This issue Cite
Research Paper
Human LAP+GARP+FOXP3+ regulatory T cells attenuate xenogeneic graft versus host disease
1. School of Public Health, China Medical University, Shenyang, China
2. Pediatrics, UTHealth Medical School, Houston, TX, USA
3. Pediatrics, Children's Cancer Hospital, University of Texas MD Anderson Cancer Center, Houston, TX, USA
4. Ivana Türbachova Laboratory for Epigenetics, Epiontis GmbH, Precision for Medicine Group, Rudower Chaussee 29, 12489 Berlin, Germany
Abstract
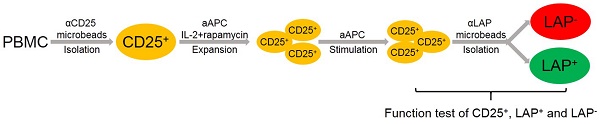
Adoptive transfer of regulatory T cells (FOXP3+ Tregs) has been developed as a potential curative immune therapy to prevent and treat autoimmune and graft-versus-host diseases (GVHD). A major limitation that has hindered the use of Treg immunotherapy in humans is the difficulty of consistently isolating and obtaining highly purified Tregs after ex vivo expansion.
Methods: We isolated bona fide Tregs from expansion cultures based on their selective surface expression of latency-associated peptide (LAP). The TCR Vβ diversity and intracellular cytokine production of Tregs were determined by flow cytometer. The TSDR methylation was determined by epigenetic human FOXP3 qPCR Assay. Their in vitro and in vivo potency was confirmed with suppression assay and humanized xenogeneic GVHD (xGVHD) murine model, respectively.
Results: LAP+ repurification results in >90% LAP+FOXP3+ Tregs, leaving behind FOXP3- and FOXP3+ nonTregs within the LAP- population. After 4-week expansion, the LAP+ Tregs were >1 billion cells, highly suppressive and anergic in vitro, >90% demethylated in the TSDR and able to maintain TCR Vβ diversity. In the xGVHD model, exogenous CD25-PBMC administered alone results in a median survival of 32 days. The co-transfer of LAP+ Tregs increased median survival to 47 days, while the LAP parent (CD25+) and LAP- nonTregs had median survival of 39 and 31 days, respectively.
Conclusions: These preclinical data together provide evidence that LAP+ Tregs are highly purified with fully suppressive function for cell therapy. This population results in a more effective and safer product for immunotherapy to treat GVHD and provides the necessary preclinical data for transition into a clinical trial with LAP+ Tregs to prevent or treat GVHD and other autoimmune diseases.
Keywords: regulatory T cells (Tregs), latency-associated peptide (LAP), Treg-specific demethylated region (TSDR), T cell receptor (TCR) repertoire, Graft-versus-host disease (GVHD)
 Global reach, higher impact
Global reach, higher impact