13.3
Impact Factor
Theranostics 2019; 9(7):2115-2128. doi:10.7150/thno.30009 This issue Cite
Research Paper
Agonist c-Met Monoclonal Antibody Augments the Proliferation of hiPSC-derived Hepatocyte-Like Cells and Improves Cell Transplantation Therapy for Liver Failure in Mice
1. State Key Laboratory of Molecular Vaccinology and Molecular Diagnostics, National Institute of Diagnostics and Vaccine Development in Infectious Diseases, School of Public Health, Xiamen University, Xiamen, 361102, PR China
2. National Institute of Diagnostics and Vaccine Development in Infectious Disease, School of Life Science, Xiamen University, Xiamen, 361102, PR China
3. Center for Molecular Imaging and Translational Medicine, School of Public Health, Xiamen, University, Xiamen 361102, China
4. Department of Microbiology, Howard University College of Medicine, Washington, DC 20059, USA
*These authors contributed equally to this work.
Received 2018-9-17; Accepted 2018-12-24; Published 2019-4-6
Abstract
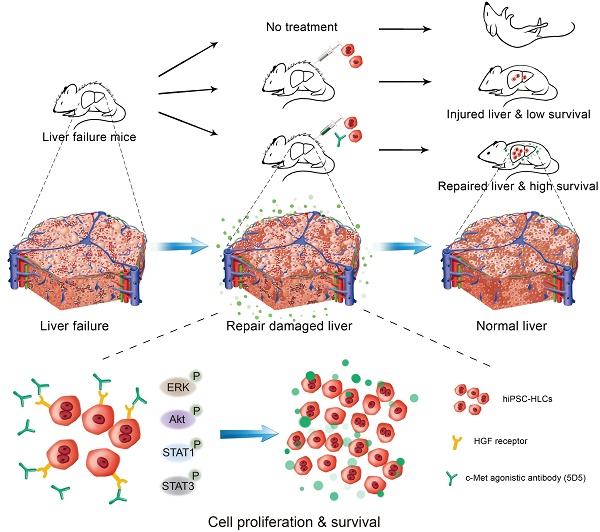
Rationale: Hepatocyte-like cells (HLCs) derived from human induced pluripotent stem cells (hiPSCs) have been developed to address the shortage of primary human hepatocytes (PHHs) for therapeutic applications. However, the in vivo repopulation capacity of HLCs remains limited. This study investigated the roles of agonist antibody activating the c-Met receptor in promoting the in vivo proliferation and repopulation of engrafted PHHs and/or HLCs in mice with liver injuries due to different causes.
Methods: An agonist c-Met receptor antibody (5D5) was used to treat PHHs and hiPSC-HLCs in both cell culture and hepatocyte-engrafted immunodeficient mice mimicking various inherited and acquired liver diseases. The promoting roles and potential influence on the hepatic phenotype of the 5D5 regimen in cell transplantation-based therapeutic applications were systematically evaluated.
Results: In hiPSC-HLC cell cultures, 5D5 treatment significantly stimulated c-Met receptor downstream signalling pathways and accelerated cell proliferation in dose-dependent and reversible manners. In contrast, only slight but nonsignificant promotion was observed in 5D5-treated PHHs. In vivo administration of 5D5 greatly promoted the expansion of implanted hiPSC-HLCs in fumarylacetoacetate hydrolase (Fah) deficient mice, resulting in significantly increased human albumin levels and high human liver chimerism (over 40%) in the transplanted mice at week 8 after transplantation. More importantly, transplantation of hiPSC-HLCs in combination with 5D5 significantly prolonged animal survival and ameliorated liver pathological changes in mice with acute and/or chronic liver injuries caused by Fas agonistic antibody treatment, carbon tetrachloride treatment and/or tyrosinemic stress.
Conclusion: Our results demonstrated that the proliferation of hiPSC-HLCs can be enhanced by antibody-mediated modulation of c-Met signalling and facilitate hiPSC-HLC-based therapeutic applications for life-threatening liver diseases.
Keywords: agonist c-Met receptor, liver failure, cell transplantation, hiPSC-derived hepatocyte-like cells, augmentation of hepatocyte proliferation
Introduction
Acute liver failure (ALF) and end-stage liver disease (ESLD) are lethal diseases with hepatocellular dysfunction, deterioration of liver functions and a high risk of mortality [1-3]. Orthotopic liver transplantation (OLT) is currently viewed as one of the most effective treatment options for ALF and ESLD, but donor organ shortages, contraindications and irreversible hepatic encephalopathy result in the death of many patients awaiting liver transplantation [4]. Therefore, alternative therapeutic strategies for ALF and ESLD are greatly needed [5]. Hepatocyte transplantation (HCTx) provides a promising alternative, and numerous studies have demonstrated that hepatocyte transplantation improves liver function in animals with liver failure and innate liver-based metabolic disorders, as well as in some clinical human studies [6-10]. However, the general problems facing HCTx are the scarcity of available donor hepatocytes and limited repopulation capacity of engrafted cells. Primary human hepatocytes (PHHs) rapidly lose their mature hepatic differentiated state and cannot be effectively expanded in vitro, and the shortages of available PHHs cannot fulfil the large number of functional hepatocytes required in HCTx-based therapy, necessitating the development of alternative cell sources [8-11]. The development of human induced pluripotent stem cells (hiPSCs) with a high differentiation potential provided a new source for hepatocyte generation not only for drug discovery but also for HCTx therapy. Several studies had successfully generated HLCs from hiPSCs via various differentiation approaches [12-14]. Because hiPSC-derived HLCs (hiPSC-HLCs) can be produced on a large scale and are directly generated from patients, they are expected to be clinically used for HCTx. However, even in an immunodeficient animal model, hiPSC-HLCs currently repopulate transplanted livers less efficiently than PHHs [13]. The limited engraftment efficiency of hiPSC-HLCs highlights the importance of a novel promotion strategy for the in vivo expansion of hiPSC-HLCs.
The c-Met protein is a transmembrane tyrosine kinase that binds hepatic growth factor (HGF). The importance of HGF/c-Met signalling during liver development and regeneration has been well demonstrated [15-17]. A recent study found that HGF secreted from transplanted hiPSC-HLCs could protect hepatocytes against cell death and increases survival in ALF mice [18]. Jin et al. reported that mouse bone marrow mononuclear cell transplantation combined with HGF administration improved both functional and histological liver recovery in carbon tetrachloride (CCl4)-injured mice [19]. These results suggested that activating HGF/c-Met signalling may improve the therapeutic effects of hiPSC-HLC transplantation. However, the short half-life (<10 minutes) of HGF limits its therapeutic application [20]. Agonist c-Met monoclonal antibody (mAb) is an alternative HGF/c-Met signalling activator with a significantly longer in vivo half-life. A previous study revealed agonist c-Met mAb could prolong the survival of transplanted PHHs in mice [21]. However, little is known about the effects and influence of agonist c-Met mAb on hiPSC-HLC transplantation-based therapy for lethal liver diseases.
Here, we performed a proof-of-concept study to investigate whether activating HGF/c-Met signalling by an agonist c-Met mAb 5D5 can improve the therapeutic performance of hepatocyte transplantation in animal models. We first evaluated the pro-proliferation effects and potential phenotypic influence of agonist c-Met mAb treatment on PHHs and hiPSC-HLCs in cell culture. Thereafter, we investigated the effects of the in vivo administration of 5D5 on promoting the expansion of PHHs and hiPSC-HLCs in fumarylacetoacetate hydrolase Fah-/-Rag2-/- IL2-/-SCID (FRGS) mice. Furthermore, we assessed the therapeutic potential of c-Met mAb in combination with cell transplantation in mice with lethal liver diseases induced by JO2 Fas/CD95 antibody, CCl4 and Fah-deficiency-related liver damage.
Methods
Generation and culture of hiPSC-HLCs
Different human induced pluripotent stem cell lines (hiPSCs named GZF2C6 induced from human fibroblasts, hiPSCs named UE005C1 induced from human urethral epithelial cells and hiPSCs named iPSN-006 induced from human amniotic mesenchymal cells) were obtained from the Key Laboratory of Regenerative Biology, Chinese Academy of Sciences (Guangzhou, China) and CELL INSPIRE BIO (Shenzhen, China) were cultured as previously described [22]. The hiPSC-HLCs derived from hiPSCs named GZF2C6 were used in all of the animal study. The hepatic differentiation of hiPSCs was performed following a three-step protocol as described in our previous study [23]. To maintain the hiPSC-HLCs in the differentiated hepatic state, they were cultured in basic Williams' Medium E (WME; GIBCO; #A1217601) with 10% foetal bovine serum (FBS; GIBCO; #10270-106), 1% dimethyl sulphoxide (DMSO; SIGMA-ALDRICH; #D2650), 10-7 M dexamethasone (DEX; LONZA; #CC4182-1), 5×10-5 M hydrocortisone (HC; LONZA; #CC-4335BB), 5 μg/mL of insulin (LONZA; #CC-4321BB) and 5 μg/mL of FH1 (APExBIO; #BRD-K4477).
Ethics Statement
All animal experiments were carried out in strict compliance with the Animal Welfare Act, PHS Policy and standards of the American Association for the Accreditation of Laboratory Animal Care and other national statutes and regulations relating to animals. The animal use protocol was approved by the Institutional Animal Care and Use Committee (IACUC) and Laboratory Animal Management Ethics Committee at Xiamen University (Protocol Number: XMULAC20160049).
Animal study
To obtain the FRGS mice, Fah-/-Rag2-/-IL-2Rγc-/- (FRG) mice as described in our previous studies [24, 25] were crossed with SCID mice (Shanghai SLAC Laboratory Animal Co., Ltd, China). The FRGS mice were bred in a specific pathogen-free (SPF) laboratory at Animal Centre of Xiamen University. The time points “day 0” and “week 0” for blood collection were at time point of 2 hours before cell transplantation. To protect the FRGS mice from Fah-/--induced liver injury, 7.5 mg/mL of 2-(2-nitro-4-trifluoro-methylbenzoyl)-1,3-cyclo-hexanedione (NTBC; SOBI, Sweden; #66607-1005-6) was added to the drinking water.
Cell transplantation
The mice were anaesthetized by isoflurane (RWD Life Science, Shenzhen, China; #R510-22) during the cell transplantation procedure. Freshly thawed PHHs or freshly digested hiPSC-HLCs of the indicated amount (1-3×106 cells) were suspended in 200 μL of basic WME and were transplanted to the mouse liver by splenic injection. The control groups received a sham operation of 200 μL of basic WME splenic injection without cells.
Statistical analysis
Student's unpaired two-tailed t-test and one-way ANOVA were performed using GraphPad Prism 7.0 (GraphPad Software). Data are presented as the means ± SD. Two-sided p-values <0.05 were considered significant: *P <0.05, **P <0.01, ***P <0.001, NS indicates no significance; U.D. indicates undetectable.
More experimental details concerning generation of hiPSC-HLCs stably expressing luciferase (Luc-hiPSC-HLCs), culture of PHHs, collection and purification of agonist c-Met mAb 5D5, NTBC withdrawal, JO2 and CCl4 induced liver failure, 5D5 treatment, enzyme-linked immunosorbent assay (ELISA), quantitative reverse transcription-polymerase chain reaction (qRT-PCR), immunofluorescence (IF) staining, cell proliferation assay, Western blotting, liver perfusion, fluorescence-activated cell sorting (FACS), immunohistochemistry (IHC) staining, luciferase detection and imaging, measurement of liver functional markers, haematoxylin and eosin (H&E) staining, (Masson's trichrome) M&T staining, reagents, antibodies and primers used in this study are provided in the Supplementary Material section.
Results
Influence of agonist c-Met mAb on the in vitro proliferation capacity of PHHs and hiPSC-HLCs
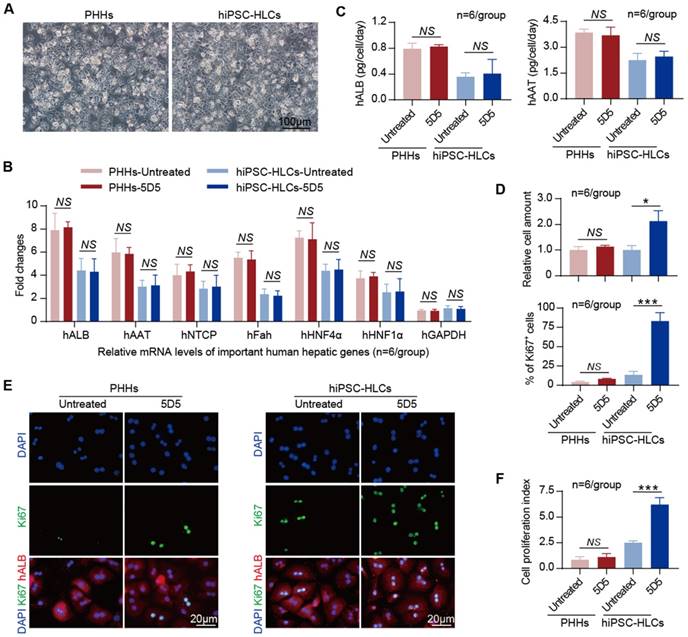
The 5D5 mAb as previously described was used as c-Met agonist in our study [21]. PHHs and hiPSC-HLCs (derived from hiPSCs GZF2C6) were used for the in vitro evaluation of 5D5 treatment. The hiPSC-HLCs were generated from hiPSCs by a traditional three-step procedure including endoderm priming, hepatic specification and maturation within 14 days [23]. The hiPSC-HLCs showed hepatocyte-like morphology, expression of hepatic-specific genes and synthesis of important hepatic proteins similar to PHHs (Fig. 1 A, B and C). Notably, the hiPSC-HLCs expressed c-Met at significantly higher levels than PHHs (Supplementary Fig. S1A). The PHHs and hiPSC-HLCs were seeded equally and treated with 0.5 mg/mL of 5D5 for 2 days, and then the hepatic phenotypes and cell proliferation capacities were measured. The qRT-PCR results showed no change in the typical hepatic-specific gene mRNA levels, including human albumin (hALB), human alpha-1-antitrypsin (hAAT), human sodium taurocholate co-transporting polypeptide (hNTCP), human Fah (hFah), human hepatocyte nuclear factor 4 alpha and 1 alpha (hHNF4α and hHNF1α) in PHHs or hiPSC-HLCs after 5D5 treatment (Fig. 1B). The ELISA quantification of hALB and hAAT levels in cell culture supernatants also showed no significant difference between the 5D5-treated and untreated control groups, either for PHHs or hiPSC-HLCs (Fig. 1C). In contrast to the controls, 5D5 treatment induced cell number increases of 1.13±0.03-fold (p=0.199) and 2.12±0.24-fold (p=0.012) in PHHs and hiPSC-HLCs, respectively (Fig. 1D, top). Moreover, IF staining for the cell proliferation marker Ki67 showed a significant increase in the Ki67-positive rate in hiPSC-HLCs after 5D5 treatment, whereas no significant change in Ki67 expression was found in 5D5-treated PHHs (Fig. 1 D, bottom and E). The cell proliferation index measured by iCELLigence further confirmed that 5D5 significantly enhanced the proliferation capacity of hiPSC-HLCs but not that of PHHs (Fig. 1F). Additionally, 5D5 accelerated cell proliferation of hiPSC-HLCs derived from different hiPSCs lines (UE005C1 and iPSN-006) with little influence on the mRNA levels of several live-specific genes and did not change the secretions of hALB and hAAT (Supplementary Fig. S1 B, C and D). Taken together, these results demonstrated that 5D5 improved the in vitro cell proliferation capacity of hiPSC-HLCs derived from three different hiPSCs lines with a minor influence on its hepatic phenotype. By contrast, 5D5 showed little effect on PHHs.
Dose-dependent and reversible activation of c-Met signalling by 5D5
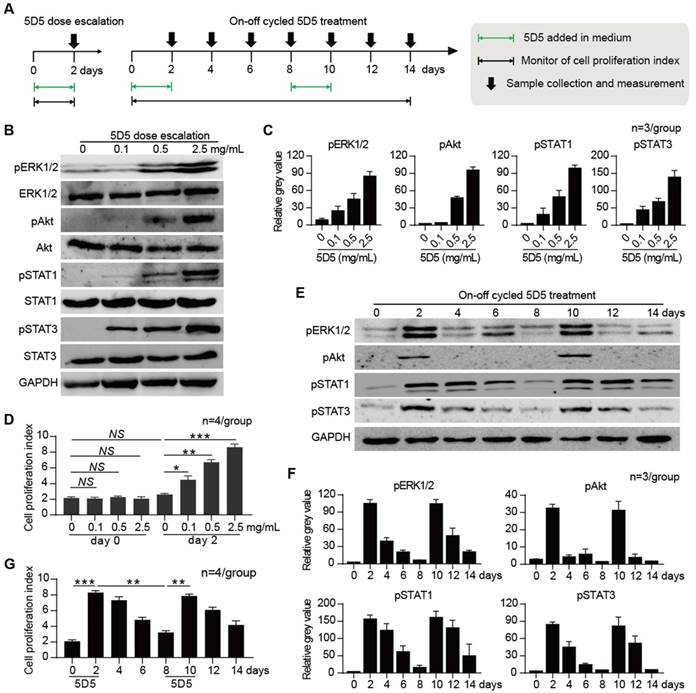
To investigate the modulation pattern of 5D5, in vitro-cultured hiPSC-HLCs (derived from hiPSCs GZF2C6) were subjected to dose escalation and on-off cycle mAb treatment for three independent experiments, respectively (Fig. 2A). The dose escalation experiment of 5D5 treatment (from 0.1 to 2.5 mg/mL) revealed that the pro-proliferation activities and phosphorylation of downstream cascade members, including extracellular signal regulated kinase (ERK), serine/threonine-specific protein kinase encoded by the Akt gene (Akt), signal transducer and activator of transcription 1 (STAT1) and STAT3, were dose dependent (Fig. 2 B, C, D and Supplementary Fig. S2A). The on-off cycle 5D5 treatment experiment revealed that the inductions of the cell proliferation index and phosphorylation of downstream cascade members were only noted during the 5D5-on period and were rapidly reduced during the 5D5-off period (Fig. 2 E, F, G and Supplementary Fig. S2B). These results demonstrated that the enhanced cell proliferation and activation of downstream kinases induced by agonist c-Met mAb are reversible and steerable.
Characterization of the promoting effect of agonist c-Met mAb 5D5 on PHHs and hiPSC-HLCs cultured in vitro. (A) Morphology of PHHs and hiPSC-HLCs cultured in vitro (bar=100 μm). (B) The relative mRNA levels of important human hepatic genes in PHHs and hiPSC-HLCs treated or untreated with 5D5 for two days were measured by qRT-PCR (n=6/group). (C) The hALB and hAAT levels in cell culture supernatants with or without 5D5 treatment were measured by ELISA (n=6/group). (D) Relative cell numbers of PHHs and hiPSC-HLCs with or without 5D5 treatment, as measured by FACS (top, n=6/group), and statistics of Ki67 expressions in different views (bottom, n=6/group). (E) Typical images of IF staining for Ki67 expression in PHHs and hiPSC-HLCs with or without 5D5 treatment (bar=20 μm). (F) Cell proliferation index of PHHs and hiPSC-HLCs cultured in vitro at two days after 5D5 treatment; the untreated cells were set as the control (n=6/group). (*P <0.05, ***P <0.001. NS indicates no significance).
Dose escalation and on-off cycle agonist c-Met mAb 5D5 treatment studies in hiPSC-HLCs cultured in vitro. (A) Schematic design of dose escalation 5D5 treatment for two days and on-off cycle 5D5 treatment for 14 days in hiPSC-HLCs cultured in vitro. (B) Representative western blot assays for the expression of down-stream proteins, including ERK, Akt, STAT1 and STAT3, and their phosphorylation activation in hiPSC-HLCs that received two-day dose escalation 5D5 treatment (from left to right: 0, 0.1, 0.5 and 2.5 mg/mL). (C) Relative grey value of the protein bands quantified by ImageJ (n=3/group). (D) Cell proliferation index of hiPSC-HLCs before and after the two-day dose escalation 5D5 treatment (top, n=4/group), and hiPSC-HLCs during the 14-day on-off cycle 5D5 treatment (bottom, n=4/group). (E) Representative western blot assays for phosphorylation activation of down-stream proteins in hiPSC-HLCs that received a 14-day on-off cycle 5D5 treatment (2.5 mg/mL of 5D5 was given from day 0 to 2 and day 8 to 10). (F) Relative grey value of the protein bands quantified by ImageJ (n=3/group). (G) Cell proliferation index of hiPSC-HLCs during the 14-day on-off cycle 5D5 treatment (bottom, n=4/group). (*P <0.05, **P <0.01, ***P <0.001. NS indicates no significance).
5D5 facilitates the in vivo expansion of hiPSC-HLCs in FRGS mice
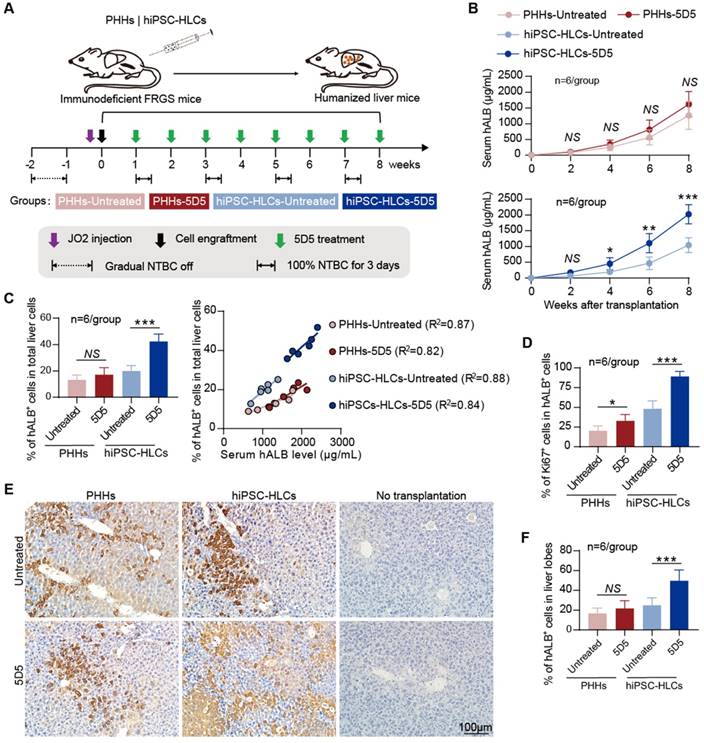
To investigate the in vivo effect of 5D5, PHHs and hiPSC-HLCs (derived from hiPSCs GZF2C6) were respectively transplanted into FRGS mice by splenic injection as previously described [23]. Cell transplantation was performed at day 7 post NTBC withdrawal. The mice were cycled on NTBC water for a course of 3 days per 2 weeks to avoid Fah-deficiency-related liver injury before Fah-functional hepatocyte engraftment. The PHH- or hiPSC-HLC-transplanted mice received weekly treatment of 5D5 (0.5 mg/kg) by intraperitoneal injection from weeks 1 to 8 after transplantation (Fig. 3A). The serum hALB levels, which indicate the in vivo expansion of implanted human hepatocytes, were quantitatively measured by ELISA, and the serum hALB level of each animal was presented in Supplementary Fig. S3. For the PHH-transplanted groups, the 5D5-treated mice showed an average serum hALB level of 1619.8±163.7 μg/mL, slightly higher (p=0.174) than that of the untreated group (1264.2±179.8 μg/mL) (Fig. 3B, top). By contrast, 5D5-treated mice with hiPSC-HLC transplantation showed an average serum hALB level of 2026.9±124.1 μg/mL, which was significantly higher (p<0.001) than that of the control group (Fig. 3B, bottom).
For further evaluations, the liver cells and tissues of animals were collected at week 8 after transplantation. The percentile of hALB-positive (hALB+) cells in collagenase-perfused liver cells or formaldehyde-fixed liver lobes were determined by FACS and IHC. For the PHH-transplanted groups, the 5D5-treated mice showed 17.1±2.2% of human liver chimerism on average at week 8 after transplantation, whereas it was 13.2±1.6% in untreated mice (Fig. 3C, left). For the hiPSC-HLC-transplanted groups, the 5D5-treated mice showed an average human liver chimerism of 42.3±2.4% at week 8 after transplantation, which was significantly higher (p<0.001) than that of untreated mice (19.9±1.7%) (Fig. 3C, left). The raw data of FACS gating scheme and cell population percentages (%) of each sample were shown in Supplementary Fig. S4A and B. Correlation analysis revealed a well correlation between the serum hALB levels and intrahepatic hALB+ percentage in both 5D5-treated and control mice that received either PHHs or hiPSC-HLCs (Fig. 3C, right). Moreover, hALB+ cells isolated from hiPSC-HLC-transplanted mice that received 5D5 showed a significantly higher Ki67-positive percentage than those in the other three groups (Fig. 3D and Supplementary Fig. S4C) but showed a similar level of hepatic-specific gene expression (Supplementary Fig. S5).
Robust expansion of hiPSC-HLCs in FRGS mice with agonist c-Met mAb 5D5 treatment. (A) Schematic design of PHH and hiPSC-HLC transplantation in FRGS mice with JO2 and gradual NTBC withdrawal-induced mild liver injury, with or without weekly c-Met agonistic antibody 5D5 treatment. (B) Serum hALB levels of FRGS mice that received PHH and hiPSC-HLC transplantation with or without agonist c-Met mAb 5D5 treatment from week 0 to 8 after transplantation (n=6/group). (C) The transplanted mice liver cells were perfused with collagenase and were analysed by FACS for the ratio of the hALB+ cells at week eight after transplantation (left, n=6/group) and linear regression analysis of the relationship between the serum hALB level and ratio of hALB+ cells in total liver cells (right, n=6/group). (D) Statistics of IF staining for the hALB+ cells collected from the PHH- and hiPSC-HLC-transplanted mice with or without agonist c-Met mAb 5D5 treatment at week eight after transplantation (n=6/group). (E) Typical images of IHC staining for the number and distribution of hALB+ cells in the PHH- and hiPSC-HLC-transplanted mice with or agonist c-Met mAb 5D5 treatment at week eight after transplantation (bar=100 μm), and (F) Statistics of IHC staining for IHC staining in different views (n=6/group). (*P <0.05, **P <0.01, ***P <0.001. NS indicates no significance).
Visualization of the intrahepatic proliferation of implanted Luc-hiPSC-HLCs. (A, B) Living images of the luciferase signal of Luc-hiPSC-HLC-transplanted mice with or without 5D5 treatment at weeks 1 and 8 after transplantation. Mice without transplantation were set as controls. (C) Total luciferase signal in the liver areas of Luc-hiPSC-HLC-transplanted mice with or without 5D5 treatment at weeks 1 and 8 after transplantation (n=5/group). (**P <0.01, NS indicates no significance; U.D. indicates undetectable).
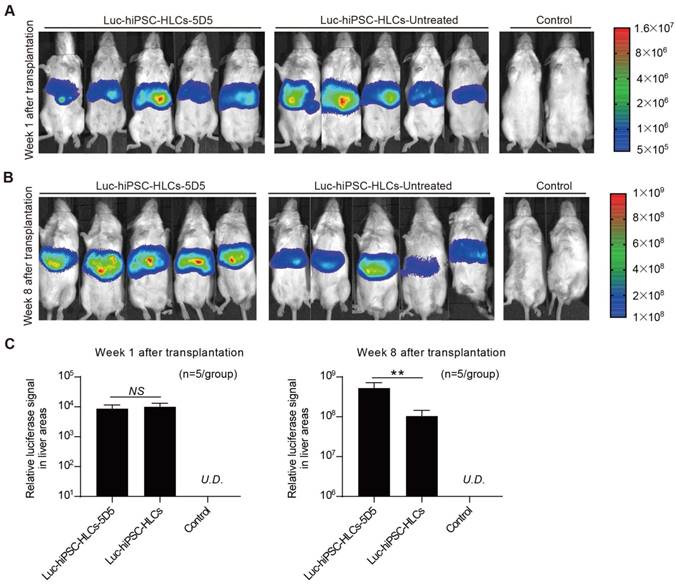
Additionally, 5D5-induced robust expansion of hiPSC-HLCs in the mouse liver was also demonstrated to be reversible (Supplementary Fig. S6). Furthermore, IHC staining for hALB+ cells in the liver lobes displayed that 5D5 treatment significantly improved the chimerism of implanted hiPSC-HLCs but has little effect on implanted PHHs (Fig. 3 E, F). To directly visualize cell proliferation in vivo, we constructed Luc-hiPSC-HLCs and transplanted the cells into FRGS mice following the same protocol in Fig. 3A. All hiPSC-HLC-transplanted mice showed similar luciferase signals in the liver areas before 5D5 treatment (week 1 after transplantation) (Fig. 4A and Fig. 4C, left). By contrast, significantly higher luciferase signals were observed in 5D5-treated mice at week 8 after transplantation (Fig. 4B and Fig. 4C, right). In summary, these results demonstrated that the 5D5 regimen significantly facilitated the in vivo expansion of functional hiPSC-HLCs in FRGS mice.
Safety profiling of 5D5 treatment in mice
We performed several measurements to characterize the safety profile of 5D5 administration in mice that received hepatocyte transplantation. First, the body weights of mice in all groups were not significantly altered (Supplementary Fig. S7A). Second, there was no significant difference regarding mouse serum biochemistry of liver function markers, including alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TBIL), bile acid (TBA), total protein (TP) and prothrombin time (PT), in the 5D5-treated groups (Supplementary Fig. S7B). Third, tumourigenicity assays including serological analysis, qRT-PCR assays for liver tissues, and IHC and H&E staining of main organs showed that no tumorigenesis was found in the mouse liver and other main organs, including the heart, liver, spleen, lung, kidney and colon (Supplementary Fig. S8). Therefore, these results suggested a favourable safety profile of hiPSC-HLC transplantation in combination with 5D5 in mice.
hiPSC-HLC transplantation in combination with 5D5 rescues mice with ALF caused by JO2
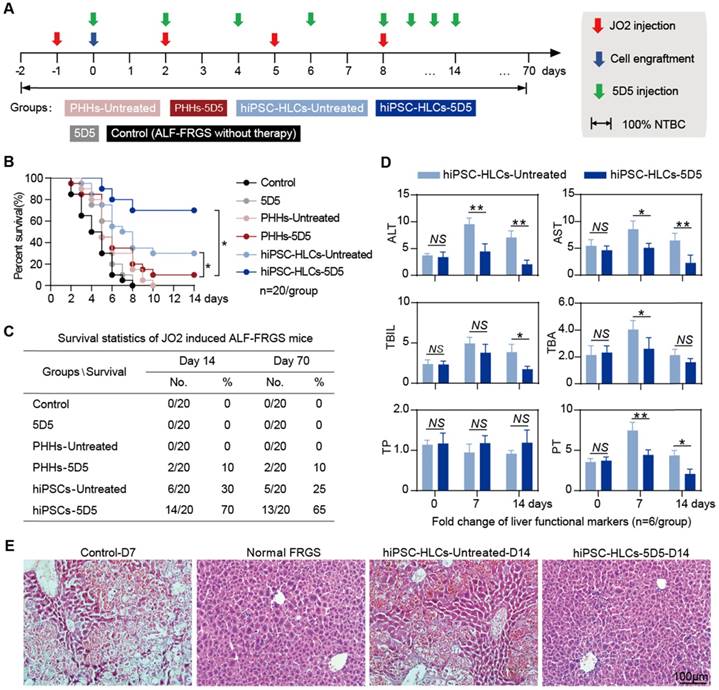
Previous studies have demonstrated that an overdose of JO2 administration induced widespread mouse hepatocyte apoptosis and caused life-threatening ALF [26-30]. Therefore, we induced life-threatening ALF in FRGS mice by multiple intraperitoneal injection of JO2 every three days. The ALF-FRGS mice received hiPSC-HLC transplantation (3×106 cells) the day after the initial JO2 injection with or without daily agonist c-Met mAb 5D5 treatment (Fig. 5A). To reduce Fah-/--induced liver injury, NTBC was maintained in the drinking water of all mice during this course. All the ALF-FRGS mice without treatment died within 8 days (Fig. 5 B, C). 5D5 regimen lacking cell transplantation did not show any therapeutic effect (Fig. 5 B, C). For the PHH transplantation groups, only 2 of 20 (10%) mice with the 5D5 regimen survived at day 14 after transplantation, but none survived at day 70 after transplantation (Fig. 5 B, C). For the hiPSC-HLC transplantation groups, 14 of 20 (70%) mice with 5D5 treatment and 6 of 20 (30%) animals without 5D5 treatment were rescued at day 14 after transplantation (Fig. 5 B, C). Moreover, 13 of 20 (65%) mice with 5D5 treatment and 5 of 20 (25%) animals without 5D5 treatment were rescued at day 70 after transplantation (Fig. 5 B, C). In contrast to the survived ALF-FRGS mice that received only hiPSC-HLC transplantation, the liver function markers ALT, AST, TBIL, TBA, TP and PT were restored to near baseline levels by hiPSC-HLC transplantation combined 5D5 treatment from days 7 to 14 after cell transplantation (Fig. 5D).
Rescuing mice with life-threatening ALF by hiPSC-HLC transplantation combined with agonist c-Met mAb 5D5 treatment. (A) Schematic design of PHH and hiPSC-HLC transplantation rescuing FRGS mice with multiple JO2 injections that induced life-threatening ALF, with or without agonist c-Met mAb 5D5 treatment. (B, C) Survival rate of the ALF-FRGS mice that received PHH and hiPSC-HLC transplantation with or without agonist c-Met mAb 5D5 treatment within 14 days after cell transplantation and a 70-day long-term observation of the survived mice (n=20/group). (D) Liver functional markers of the survived mice that received hiPSC-HLC transplantation with or without agonist c-Met mAb 5D5 treatment and normal FRGS mice without induced ALF within 14 days (n=6/group). (E) H&E staining of the liver tissues collected from the survived ALF-FRGS mice with or without agonist c-Met mAb 5D5 treatment at day 14 after transplantation. Liver tissues collected from the deceased ALF-FRGS mice without cell transplantation or antibody treatment (Control-D7) and normal FRGS mice are also shown (bar=100μm). (*P <0.05, **P <0.01. NS indicates no significance).
H&E staining further confirmed that hiPSC-HLC transplantation combined with 5D5 was notably coupled with repair of the damaged liver structure at day 14, whereas the deceased mice with only hiPSC-HLC transplantation or untreated mice showed a typical ALF histology with extensive hepatic apoptosis and haemorrhage in varied degrees (Fig. 5E). The serum hALB level of the survived mice with hiPSC-HLC transplantation combined with 5D5 was 522.1±22.8 μg/mL at day 14 after transplantation and was maintained for at least 70 days, indicating ~10% of mouse hepatocytes were replaced by the implanted hiPSC-HLCs (Supplementary Fig. S9A). However, the survived mice with only hiPSC-HLC transplantation achieved 252.1±22.8 mg/mL at day 14 after transplantation, indicating ~5% of liver chimerism (Fig. 5F). In addition, 5D5 treatment significantly increased the population of Ki67+ cells in hALB+ cells that perfused from FRGS mice liver at day 70 after transplantation (Supplementary Fig. S9B). Taken together, these results demonstrated that hiPSC-HLC transplantation is better than PHH transplantation in ALF therapy, and the enhanced cell proliferation capacity of the implanted cells by 5D5 may be essential to rescue mice from drug-induced acute liver injury.
hiPSC-HLC transplantation in combination with 5D5 ameliorates mice with chronic liver injury
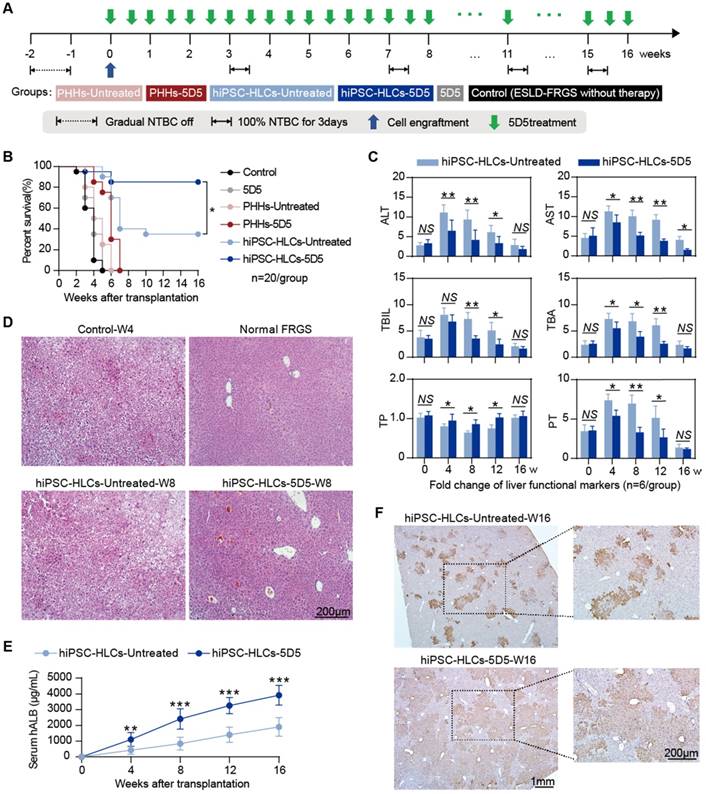
Fah-deficient mice are widely used to model life-threatening chronic liver injury via long-term NTBC withdrawal [31, 32]. Full NTBC withdrawal over 4 weeks usually cause death in FRGS mice, even for those during the early stage after normal hepatocyte transplantation. To test the therapeutic performance of hiPSC-HLC transplantation with 5D5 stimulation, we extended the NTBC-dosing interval to 4 weeks as described in Fig. 6A. According this procedure, all un-transplanted mice (control group) died within 5 weeks, and mono-5D5 administration showed no therapeutic effect (Fig. 6B). For the PHH-transplanted group, 5D5 administration did not exhibit a significant benefit to rescue mice, and all PHH-transplanted mice were lost within 8 weeks (Fig. 6B). Among the hiPSC-HLC-transplanted mice, 7 (7/20, 35%) in the non-5D5-treated group and 17 (17/20, 85%) in the 5D5-treated group achieved long-term survival for at least 16 weeks, suggesting a significant (p=0.003) promotion of 5D5 in implanted hiPSC-HLCs. The profiling of the serological markers of ALT, AST, TBIL, TBA, TP and PT suggested that 5D5 treatment significantly facilitated liver function recovery in mice with hiPSC-HLC transplantation (Fig. 6C). H&E staining further confirmed that hiPSC-HLC transplantation in combination with 5D5 treatment greatly repaired the liver structure to a nearly normal phenotype at week 16 after cell transplantation, whereas the hiPSC-HLC-transplanted mice without 5D5 administration still showed liver injury in some of the liver lobes (Fig. 6D). At week 16, the survived mice with 5D5 treatment showed an average serum hALB level of 3916.6±257.5 μg/mL, which was significantly higher than that of non-5D5-treated mice (p<0.001, Fig. 6E). Furthermore, IHC staining for hALB+ cells in mouse liver lobes at week-16 confirmed that the 5D5 regimen significantly improved implanted human cell chimerism (Fig. 6F). The average percentage of Ki67+ cells in hALB+ cells derived from 5D5-treated mice was significantly higher than non-5D5 groups at week 16 after hiPSC-HLC transplantation. (Supplementary Fig. S10A). These results demonstrated that the implanted hiPSC-HLCs robustly expanded in vivo and replaced the damaged hepatocytes with healthy ones, thereby rescuing mice with chronic liver injury induced by long-term NTBC withdrawal.
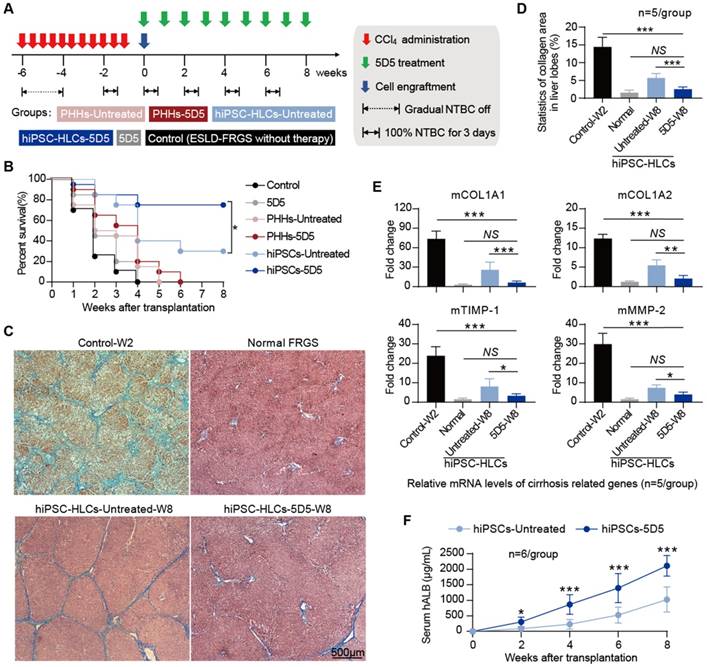
Liver fibrosis with collagen deposition and abnormal liver structure is the common result of chronic liver injury, and we subsequently evaluated the liver regenerative effect of 5D5-enhanced hiPSC-HLC transplantation in FRGS mice with chronic iterative toxic damage induced by CCl4 intoxication. Chronic liver injury was induced by NTBC cycled-off and administration of CCl4 twice per week for six weeks as previously described [25]. At week 6 after initial CCl4 administration, mice with chronic liver injury received hiPSC-HLC or PHH transplantation (3×106 cells) in combination with 5D5 (Fig. 7A). All mice without cell transplantation died within 10 weeks after initial CCl4 administration regardless of 5D5 regimen. (Fig. 7B). More than 70% (15/20) of mice that received 5D5-enhanced hiPSC-HLC transplantation survived at week 8, whereas 30% (6/20) of the mice that received hiPSC-HLC transplantation survived if the 5D5 regimen was absent (Fig. 7B). By contrast, no animal survived in the non-treated control group or received PHH transplantation (Fig. 7B). Notably, M&T staining of liver tissues showed that hiPSC-HLC transplantation in combination with 5D5 significantly reduced collagen deposition and ameliorated abnormal liver structure at week 8, and hiPSC-HLC transplantation without 5D5 treatment slightly reduced liver cirrhosis pathology (Fig. 7 C, D). Furthermore, hiPSC-HLC transplantation in combination with 5D5 treatment significantly reduced the RNA levels of mouse cirrhosis-related genes, including mCOL1A1, mCOL1A2, mTIMP-1, mMMP-2, whereas hiPSC-HLC transplantation in the absence of 5D5 only slightly decreased the expression of these genes (Fig. 7E). The mice that received hiPSC-HLC transplantation combined with 5D5 treatment showed higher serum hALB levels from week 0 to 8 after transplantation than the mice that received hiPSC-HLC transplantation without 5D5 treatment, indicating robust proliferation and a higher liver chimeric rate of the implanted hiPSC-HLCs (Fig. 7F). Consistent with the finding on other models of this study, significant increased Ki67+ cells percentage in hALB+ cells was noted in the liver of 5D5-treated mice at week eight after transplantation (Supplementary Fig. S10B). These results suggested that hiPSC-HLC transplantation in combination with 5D5 provided an improved therapeutic approach for drug-induced chronic liver injury.
Repopulation of damaged hepatocytes with implanted hiPSC-HLCs in mice with life-threatening ESLD is improved by the combination of agonist c-Met mAb treatment. (A) Schematic design of PHHs and hiPSC-HLC transplantation rescuing FRGS mice with long-term NTBC off that induced life-threatening ESLD, with or without agonist c-Met mAb 5D5 treatment. (B) Survival rate of the ESLD-FRGS mice that received PHH and hiPSC-HLC transplantation with or without agonist c-Met mAb 5D5 treatment within 16 weeks after cell transplantation (n=20/group). (C) Liver function markers of the survived mice that received hiPSC-HLC transplantation with or without agonist c-Met mAb 5D5 treatment and normal FRGS mice without induced ALF within 16 weeks after cell transplantation (n=6/group). (D) H&E staining of the liver tissues collected from the survived ESLD-FRGS mice with or without agonist c-Met mAb 5D5 treatment at week eight after transplantation. The liver tissues collected from the deceased ESLD-FRGS mice without cell transplantation or antibody treatment (Control-W4) and normal FRGS mice are also shown (bar=200 μm). (E) Serum hALB levels of the survived ESLD-FRGS mice that received hiPSC-HLC transplantation with or without agonist c-Met mAb 5D5 treatment from weeks 0 to 16 after cell transplantation (n=6/group). (F) IHC staining for the number and distribution of hALB+ cells in liver lobes collected from the survived ESLD-FRGS mice that received hiPSC-HLC transplantation with or without agonist c-Met mAb 5D5 treatment at week 16 after cell transplantation (left, bar=1 mm; right, bar=200 μm). (*P <0.05, **P <0.01, ***P <0.001. NS indicates no significance).
Combined therapy of hiPSC-HLC transplantation and agonist c-Met mAb 5D5 treatment ameliorates ESLD with liver fibrosis. (A) Schematic design of PHH and hiPSC-HLC transplantation rescuing FRGS mice with CCl4 administration and NTBC off that induced life-threatening ESLD and liver fibrosis, with or without agonist c-Met mAb 5D5 treatment. (B) Survival rate of ESLD-FRGS mice that received PHH and hiPSC-HLC transplantation with or without agonist c-Met mAb 5D5 treatment within eight weeks after cell (n=20/group). (C) M&T staining of liver tissues collected from the survived ESLD-FRGS mice with or without agonist c-Met mAb 5D5 treatment at week eight after transplantation. The liver tissues collected from the deceased ESLD-FRGS mice without cell transplantation or antibody treatment (Control-W2) and normal FRGS mice are also shown (bar=500 μm). (D) Statistics of the collagen area (stained blue) in liver lobes (n=5/group). (E) Relative mRNA levels of cirrhosis-related genes in liver tissues collected from the survived ESLD-FRGS mice with or without agonist c-Met mAb 5D5 treatment at week eight after transplantation. Liver tissues collected from the deceased ESLD-FRGS mice without cell transplantation or antibody treatment (Control-W2) and normal FRGS mice were also detected (n=5/group). (F) Serum hALB levels of the survived ESLD-FRGS mice that received hiPSC-HLCs transplantation with or without agonist c-Met mAb 5D5 treatment from weeks 0 to 8 after cell transplantation (n=6/group). (*P <0.05, **P <0.01, ***P <0.001. NS indicates no significance).
Discussion
Liver transplantation is currently regarded as the only clinical treatment option for ALF and ESLD. However, the requirements for liver transplantation were greatly outstripped by the supply of donor organs. In the past decades, HCTx-based therapies have been developed to be an alternative to whole-liver transplantation, but it remains limited by the inadequate cell source of the transplanted hepatocytes and poor repopulation capacity of the engrafted cells [11]. Recently, in vitro-differentiated HLCs from embryonic stem cells (ESCs) [13], mesenchymal or matrix stem cells (MSCs) [33], directly reprogrammed human fibroblasts [34] and hiPSCs [12, 23] have been proposed as alternative sources of PHHs for HCTx-based therapies. Previous studies have demonstrated that the transplantation of a clinical scale (approximately 3×107 cells) of human bone-derived MSCs via the intrahepatic portal vein under B-ultrasound guidance could effectively rescue large animals (pigs) with fulminant hepatic failure [35, 36]. Human HLCs generated from direct programming from human hepatoblasts also showed a therapeutic effect on ALF pigs though a bioartificial liver device (BAL) [34]. Furthermore, hiPSCs reprogrammed from somatic cells by the overexpression of pluripotency factors provided an unlimited source to produce HLCs for BAL- and HCTx-based therapies in theory [37]. Recent studies have indicated that hiPSC-derived HLCs are partially functionally equivalent to PHHs and are potentially applicable both in clinical and drug discovery studies [13, 37-40]. Compared with other cell sources, hiPSCs have the characteristic to proliferate infinitely, enabling scaling up bioprocesses to obtain adequate functional HLCs cells for clinical use [38, 41]. Moreover, hiPSC-HLCs can be customized from a patient's somatic cells to fit various genetic backgrounds, providing the possibility of generating HLCs from the patient's own cells for transplantation without further immunosuppression therapy [12, 13].
Although the many in vitro differentiated HLCs derived from hiPSCs and other human stem cells were demonstrated to be functional and expandable in vivo, the engraftment and expansion of these HLCs were still less efficient, as reflected by the much lower serum hALB levels of recipient animals than those that received transplantation of PHHs [42]. This limitation was possibly attributed to the inadequate in vivo proliferation capability and/or immature hepatic excretory function of implanted HLCs. Some small molecules, such as XMU-MP-1, FH1 and FPH1/2, showed the promotion effects on cell proliferation or hepatic differentiation, suggesting the potential to augment liver repair and regeneration [25, 43, 44]. However, the pharmacological and cellular mechanisms of FH1 and FPH1/2 are less understood, and the in vivo effects of these compounds have not been evaluated. The XMU-MP-1 targeting of MST1/2 kinases in the Hippo pathway showed positive results in promoting the expansion of PHHs in FRG mice but had not been tested for hiPSC-derived HLCs. In addition, as shown in previous studies, single-cell transplantation or pharmacological therapy was inadequate to rescue mice from severe liver failure conditions, such as an Fah-deficiency-induced liver injury [31]. Compared with chemical compounds, bioactive protein-based enhancers (such as growth factors and antibodies) have better biocompatibility, possibly higher activities and less safety concern. The characteristics and functions of c-Met protein, which were widely distributed on the liver cell surface, have been well demonstrated [15-17]. HGF/c-Met signalling play major roles in liver repair and regeneration by controlling autologous cell proliferation and/or providing an effective target to augment the robust expansion of the implanted cells [45-48]. As previous studies and our studies have shown, agonist c-Met mAb can mimic the signalling of HGF, activate downstream kinases and promote cell proliferation, implying the potential of agonist c-Met mAb in liver regeneration medicine [49].
Our study systematically evaluated the effects of the agonist c-Met antibody both in cell culture and mice mimicking human liver diseases requiring transplantation (summarized in the graphical abstract). For the first time, we found the agonist c-Met mAb 5D5 exhibited more potent promotion of cell proliferation in hiPSC-HLCs than in PHHs (Fig. 1 and Fig. 2). More importantly, animal studies revealed that the in vivo administration of 5D5 significantly improved therapeutic effectiveness of hiPSC-HLC transplantation in rescuing animals with inherited and acquired life-threatening liver diseases with a favourable safety profile. Evidence from survival monitoring, serological analyses and histopathological examinations simultaneously demonstrated that the 5D5 regimen greatly facilitated the in vivo expansion of hiPSC-HLCs, ameliorated liver failure, restoring liver function and repairing the damaged liver structure in animals with liver injuries induced by JO2 (Fig. 5), long-term NTBC withdrawal (Fig. 6), or CCl4 (Fig. 7). Interestingly, our results noted that hiPSC-HLC engraftment combined with 5D5 treatment ameliorated liver fibrosis progression in ESLD-FRGS mice within eight weeks, suggesting the therapeutic potential of such a strategy in treating liver fibrosis and/or cirrhosis. However, the mechanisms should be further investigated.
Conclusions
Our novel antibody-enhanced hiPSC-HLC transplantation strategy exhibited promising therapeutic effects in mice. Nonetheless, some limitations should also be noted for our study. First, the agonist c-Met mAb used in this study was mouse mAb against human c-Met receptor. Humanization of the mAb is needed for further investigation. On the other hand, the use of agonist c-Met mAb in immunocompetent human cells may have more complicated effects. Second, the slope of the correlation curve between intrahepatic human cell chimerism and mouse serum hALB levels (Fig. 3C, right) was still significantly higher in the hiPSC-HLC-transplanted groups than in the transplanted PHHs regardless of the 5D5 regimen, suggesting hiPSC-HLCs were still less efficient than PHHs in the secretion of hALB. In addition to 5D5-like accelerators for cell proliferation, improvement in the hepatic function maturation of hiPSC-HLCs may further facilitate hiPSC-HLC-based therapeutic applications. Nevertheless, our study developed a novel strategy to extend the applicable potential of hiPSC-HLCs. To promote the clinic application of our new strategy, more safety tests and therapeutic evaluations need to be performed in large animals, such as pigs and macaque monkeys. Because the current antibody industry is adequate to support clinic applications, developing clinical-scale production and quality control methods of hiPSC-HLCs is important to guarantee and promote therapeutic effect. Further investigations of our new strategy in more severe and complex liver failure conditions, including end-stage liver cirrhosis and autoimmune, alcoholic and non-alcoholic fatty liver diseases, will be interesting and challenging and will help to determine the therapeutic effects. The promoting effects of agonist c-Met c antibody on human HLCs derived from other stem cells (ESCs or MSCs) or directly reprogrammed hepatocytes will also be a promising direction of liver regeneration medicine. In summary, our work sets the stage for using cell transplantation combined with antibody therapy to rescue recipients in acute or chronic life-threatening liver failure conditions.
Abbreviations
Akt: serine/threonine-specific protein kinase encoded by the Akt gene
ALF: acute liver failure
CCl4: carbon tetrachloride
ELISA: enzyme-linked immunosorbent assay
ERK: extracellular signal regulated kinase
ESLD: end-stage liver disease
FACS: fluorescence activated cell sorting
Fah: fumarylacetoacetate hydrolase
FRG mice: Fah-/-Rag2-/-IL-2Rγc-/- mice
FRGS mice: Fah-/-Rag2-/-IL2-/-SCID mice
HCTx: hepatocyte transplantation
H&E staining: haematoxylin and eosin staining
HGF: hepatic growth factor
hiPSC-HLCs: hiPSC-derived HLCs
hiPSCs: induced pluripotent stem cells
HLCs: hepatocyte-like cells
IF staining: immunofluorescence staining
IHC staining: immunohistochemistry staining,
Luc: luciferase
Luc-hiPSC-HLCs: hiPSC-HLCs stably expressing luciferase
M&T staining: Masson's trichrome staining
mAb: monoclonal antibody
NTBC: 2-(2-nitro-4-trifluoro-methylbenzoyl)-1, 3-cyclo-hexanedione
OLT: orthotopic liver transplantation
PHHs: primary human hepatocytes
qRT-PCR: quantitative reverse transcription-polymerase chain reaction
STAT1: signal transducer and activator of transcription 1
STAT3: signal transducer and activator of transcription 3
5D5: agonist c-Met receptor monoclonal antibody 5D5
Supplementary Material
Supplementary figures and tables.
Acknowledgements
This work was supported by the National Science and Technology Major Projects for Major New Drugs Innovation and Development (No. 2018ZX09711003-005-003) (T.C.), the National Science and Technology Major Project of Infectious Diseases (No. 2017ZX10304402) (T.C.), the National Natural Science Foundation of China (No. 81672023, 81871316) (Q.Y.) and the President Fund of Xiamen University (No. 20720160063) (Q.Y.). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author contributions
L.Z.Y., Y.L.Z. and X.L. contributed equally to this work. L.Z.Y. and X.L. established the cell culture system. L.Z.Y., Y.L.Z. and X.L. performed antibody production and purification. L.Z.Y., Y.L.Z., X.L., L.Z., Y.C. and K.W. performed animal studies. L.Z.Y., Y.L.Z., X.L., L.Z., Y.C., J.L.C., M.F.W. and X.L.L. performed cell and blood sample measurement. L.Z.Y., X.L., L.Z. and Y.C., performed histological analysis. X.L., Y.C. and L.Z. performed animal live imaging. J.Z., Q.Y.T. and G.L. participated in the experimental design and data analysis. L.Z.Y., Y.L.Z., X.L. and Q.Y. wrote the manuscript. Q.Y., T.C. and N.S.X. supervised the project.
Competing Interests
The authors have declared that no competing interest exists.
References
1. Elias E. Liver failure and liver disease. Hepatology. 2006;43:S239-42
2. Jalan R, Gines P, Olson JC, Mookerjee RP, Moreau R, Garcia-Tsao G. et al. Acute-on chronic liver failure. J Hepatol. 2012;57:1336-48
3. Bernal W, Wendon J. Acute liver failure. N Engl J Med. 2013;369:2525-34
4. Starzl TE. The long reach of liver transplantation. Nat Med. 2012;18:1489-92
5. Viswanathan P, Gupta S. New directions for cell-based therapies in acute liver failure. J Hepatol. 2012;57:913-5
6. Lorenzo M, Angrisani L, Santoro A, Di Salvo E. [Hepatocyte transplantation. From experimentation to therapeutic attempts in man]. G Chir. 1994;15:59-63
7. Schneider A, Attaran M, Meier PN, Strassburg C, Manns MP, Ott M. et al. Hepatocyte transplantation in an acute liver failure due to mushroom poisoning. Transplantation. 2006;82:1115-6
8. Strom SC, Bruzzone P, Cai H, Ellis E, Lehmann T, Mitamura K. et al. Hepatocyte transplantation: clinical experience and potential for future use. Cell Transplant. 2006;15(Suppl 1):S105-10
9. Gewartowska M, Olszewski WL. Hepatocyte transplantation-biology and application. Ann Transplant. 2007;12:27-36
10. Akhter J, Johnson LA, Gunasegaram A, Riordan SM, Morris DL. Hepatocyte transplantation: a review of laboratory techniques and clinical experiences. Surgeon. 2007;5:155-64
11. Puppi J, Dhawan A. Human hepatocyte transplantation overview. Methods Mol Biol. 2009;481:1-16
12. Xia Y, Carpentier A, Cheng X, Block PD, Zhao Y, Zhang Z. et al. Human stem cell-derived hepatocytes as a model for hepatitis B virus infection, spreading and virus-host interactions. J Hepatol. 2017;66:494-503
13. Carpentier A, Tesfaye A, Chu V, Nimgaonkar I, Zhang F, Lee SB. et al. Engrafted human stem cell-derived hepatocytes establish an infectious HCV murine model. J Clin Invest. 2014;124:4953-64
14. Shlomai A, Schwartz RE, Ramanan V, Bhatta A, de Jong YP, Bhatia SN. et al. Modeling host interactions with hepatitis B virus using primary and induced pluripotent stem cell-derived hepatocellular systems. Proc Natl Acad Sci U S A. 2014;111:12193-8
15. Giordano S, Ponzetto C, Di Renzo MF, Cooper CS, Comoglio PM. Tyrosine kinase receptor indistinguishable from the c-met protein. Nature. 1989;339:155-6
16. Bottaro DP, Rubin JS, Faletto DL, Chan AM, Kmiecik TE, Vande Woude GF. et al. Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product. Science. 1991;251:802-4
17. Bladt F, Riethmacher D, Isenmann S, Aguzzi A, Birchmeier C. Essential role for the c-met receptor in the migration of myogenic precursor cells into the limb bud. Nature. 1995;376:768-71
18. Nagamoto Y, Takayama K, Ohashi K, Okamoto R, Sakurai F, Tachibana M. et al. Transplantation of a human iPSC-derived hepatocyte sheet increases survival in mice with acute liver failure. J Hepatol. 2016;64:1068-75
19. Jin SZ, Meng XW, Sun X, Han MZ, Liu BR, Wang XH. et al. Hepatocyte growth factor promotes liver regeneration induced by transfusion of bone marrow mononuclear cells in a murine acute liver failure model. J Hepatobiliary Pancreat Sci. 2011;18:397-405
20. Ido A, Moriuchi A, Kim I, Numata M, Nagata-Tsubouchi Y, Hasuike S. et al. Pharmacokinetic study of recombinant human hepatocyte growth factor administered in a bolus intravenously or via portal vein. Hepatol Res. 2004;30:175-81
21. Ohashi K, Marion PL, Nakai H, Meuse L, Cullen JM, Bordier BB. et al. Sustained survival of human hepatocytes in mice: A model for in vivo infection with human hepatitis B and hepatitis delta viruses. Nat Med. 2000;6:327-31
22. Esteban MA, Wang T, Qin B, Yang J, Qin D, Cai J. et al. Vitamin C enhances the generation of mouse and human induced pluripotent stem cells. Cell Stem Cell. 2010;6:71-9
23. Yuan L, Liu X, Zhang L, Li X, Zhang Y, Wu K. et al. A Chimeric Humanized Mouse Model by Engrafting the Human Induced Pluripotent Stem Cell-Derived Hepatocyte-Like Cell for the Chronic Hepatitis B Virus Infection. Front Microbiol. 2018;9:908
24. Zhang TY, Yuan Q, Zhao JH, Zhang YL, Yuan LZ, Lan Y. et al. Prolonged suppression of HBV in mice by a novel antibody that targets a unique epitope on hepatitis B surface antigen. Gut. 2016;65:658-71
25. Fan F, He Z, Kong LL, Chen Q, Yuan Q, Zhang S. et al. Pharmacological targeting of kinases MST1 and MST2 augments tissue repair and regeneration. Sci Transl Med. 2016;8:352ra108
26. Mignon A, Guidotti JE, Mitchell C, Fabre M, Wernet A, De La Coste A. et al. Selective repopulation of normal mouse liver by Fas/CD95-resistant hepatocytes. Nat Med. 1998;4:1185-8
27. Wilson JH, Paturzo FX, Johnson LK, Carreiro MP, Hixson DC, Mennone A. et al. Rat hepatocyte engraftment in severe combined immunodeficient x beige mice using mouse-specific anti-fas antibody. Xenotransplantation. 2006;13:53-62
28. Nishimura Y, Hirabayashi Y, Matsuzaki Y, Musette P, Ishii A, Nakauchi H. et al. In vivo analysis of Fas antigen-mediated apoptosis: effects of agonistic anti-mouse Fas mAb on thymus, spleen and liver. Int Immunol. 1997;9:307-16
29. Vidal I, Blanchard N, Alexandre E, Gandillet A, Chenard-Neu MP, Staedtler F. et al. Improved xenogenic hepatocyte implantation into nude mouse liver parenchyma with acute liver failure when followed by repeated anti-Fas antibody (Jo2) treatment. Cell Transplant. 2008;17:507-24
30. Bility MT, Cheng L, Zhang Z, Luan Y, Li F, Chi L. et al. Hepatitis B virus infection and immunopathogenesis in a humanized mouse model: induction of human-specific liver fibrosis and M2-like macrophages. PLoS Pathog. 2014;10:e1004032
31. Huang P, Zhang L, Gao Y, He Z, Yao D, Wu Z. et al. Direct reprogramming of human fibroblasts to functional and expandable hepatocytes. Cell Stem Cell. 2014;14:370-84
32. Yan F, Wang Y, Zhang W, Chang M, He Z, Xu J. et al. Human embryonic stem cell-derived hepatoblasts are an optimal lineage stage for hepatitis C virus infection. Hepatology. 2017;66:717-35
33. Paganelli M, Dallmeier K, Nyabi O, Scheers I, Kabamba B, Neyts J. et al. Differentiated umbilical cord matrix stem cells as a new in vitro model to study early events during hepatitis B virus infection. Hepatology. 2013;57:59-69
34. Shi XL, Gao Y, Yan Y, Ma H, Sun L, Huang P. et al. Improved survival of porcine acute liver failure by a bioartificial liver device implanted with induced human functional hepatocytes. Cell Res. 2016;26:206-16
35. Li J, Zhang L, Xin J, Jiang L, Li J, Zhang T. et al. Immediate intraportal transplantation of human bone marrow mesenchymal stem cells prevents death from fulminant hepatic failure in pigs. Hepatology. 2012;56:1044-52
36. Shi D, Zhang J, Zhou Q, Xin J, Jiang J, Jiang L. et al. Quantitative evaluation of human bone mesenchymal stem cells rescuing fulminant hepatic failure in pigs. Gut. 2017;66:955-64
37. Asgari S, Pournasr B, Salekdeh GH, Ghodsizadeh A, Ott M, Baharvand H. Induced pluripotent stem cells: a new era for hepatology. J Hepatol. 2010;53:738-51
38. Hannoun Z, Steichen C, Dianat N, Weber A, Dubart-Kupperschmitt A. The potential of induced pluripotent stem cell derived hepatocytes. J Hepatol. 2016;65:182-99
39. Takebe T, Sekine K, Enomura M, Koike H, Kimura M, Ogaeri T. et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. 2013;499:481-4
40. Takebe T, Zhang RR, Koike H, Kimura M, Yoshizawa E, Enomura M. et al. Generation of a vascularized and functional human liver from an iPSC-derived organ bud transplant. Nat Protoc. 2014;9:396-409
41. Ni Y, Urban S. Stem cell-derived hepatocytes: A promising novel tool to study hepatitis B virus infection. J Hepatol. 2017;66:473-5
42. Gao Y, Zhang X, Zhang L, Cen J, Ni X, Liao X. et al. Distinct Gene Expression and Epigenetic Signatures in Hepatocyte-like Cells Produced by Different Strategies from the Same Donor. Stem Cell Reports. 2017;9:1813-24
43. Shan J, Schwartz RE, Ross NT, Logan DJ, Thomas D, Duncan SA. et al. Identification of small molecules for human hepatocyte expansion and iPS differentiation. Nat Chem Biol. 2013;9:514-20
44. Liu Z, Li Y, Li W, Xiao C, Liu D, Dong C. et al. Multifunctional Nanohybrid Based on Porous Silicon Nanoparticles, Gold Nanoparticles, and Acetalated Dextran for Liver Regeneration and Acute Liver Failure Theranostics. Adv Mater. 2017
45. Thorgeirsson SS. The central role of the c-Met pathway in rebuilding the liver. Gut. 2012;61:1105-6
46. Kaldenbach M, Giebeler A, Tschaharganeh DF, Erschfeld S, Wasmuth HE, Dolle L. et al. Hepatocyte growth factor/c-Met signalling is important for the selection of transplanted hepatocytes. Gut. 2012;61:1209-18
47. Ishikawa T, Factor VM, Marquardt JU, Raggi C, Seo D, Kitade M. et al. Hepatocyte growth factor/c-met signaling is required for stem-cell-mediated liver regeneration in mice. Hepatology. 2012;55:1215-26
48. Huh CG, Factor VM, Sanchez A, Uchida K, Conner EA, Thorgeirsson SS. Hepatocyte growth factor/c-met signaling pathway is required for efficient liver regeneration and repair. Proc Natl Acad Sci U S A. 2004;101:4477-82
49. Comoglio PM, Giordano S, Trusolino L. Drug development of MET inhibitors: targeting oncogene addiction and expedience. Nat Rev Drug Discov. 2008;7:504-16
Author contact
![]() Corresponding author: Quan Yuan, 86-592-2880627 Fax: 86-592-2181258 email: yuanquanedu.cn. Tong Cheng, Tel: 86-592-2184113 Fax: 86-592-2181258 email: tchengedu.cn
Corresponding author: Quan Yuan, 86-592-2880627 Fax: 86-592-2181258 email: yuanquanedu.cn. Tong Cheng, Tel: 86-592-2184113 Fax: 86-592-2181258 email: tchengedu.cn
 Global reach, higher impact
Global reach, higher impact