13.3
Impact Factor
Theranostics 2019; 9(7):2036-2055. doi:10.7150/thno.32738 This issue Cite
Research Paper
UBE2C, Directly Targeted by miR-548e-5p, Increases the Cellular Growth and Invasive Abilities of Cancer Cells Interacting with the EMT Marker Protein Zinc Finger E-box Binding Homeobox 1/2 in NSCLC
1. Clinical Medical Laboratory, Binzhou Medical University Hospital, Binzhou, 256603, P.R. China
2. Cancer research institute, Binzhou Medical University Hospital, Binzhou, 256603, P.R. China
3. Department of Thyroid and Breast Surgery, Binzhou Medical University Hospital, Binzhou, 256603, P.R. China
4. Department of Clinical Laboratory, Binzhou Medical University Hospital, Binzhou, 256603, P.R. China
5. Department of Gastrointestinal Surgery, Binzhou Medical University Hospital, Binzhou, 256603, P.R. China
6. Department of Hepatobiliary Surgery, Binzhou Medical University Hospital, Binzhou, 256603, P.R. China
*These authors contributed equally to this work
Abstract
Background: Recent evidence indicates that UBE2C participates in carcinogenesis by regulating the cell cycle, apoptosis, metastasis, and transcriptional processes. Additionally, miR-548e-5p dysregulation plays a vital role in tumor progression. However, the molecular mechanism via which UBE2C is directly targeted by miR-548-5p, resulting in increase in cellular growth and invasiveness of cancer cells, and its interactions with the epithelial-mesenchymal transition (EMT) marker protein ZEB1/2 in non-small cell lung cancer (NSCLC) is not understood.
Methods: Expression of UBE2C and miR-548e-5p was analyzed using reverse transcription-quantitative polymerase chain reaction (RT-qPCR). The protein level of UBE2C and ZEB1/2 was analyzed using western blotting and immunofluorescence staining. Cellular proliferation was detected using the cell counting kit 8 (CCK8) and 3-(4,5-dimethylthiazol-2-Yl)-2,5-diphenyltetrazolium bromide (MTT) assays. Cell migration, invasion, and growth were analyzed using the wound healing and transwell assay. Promoter activity and transcription was analyzed using the luciferase reporter assay. Chromatin immunoprecipitation was used to detect binding of UBE2C to 5′UTR-ZEB1/2.
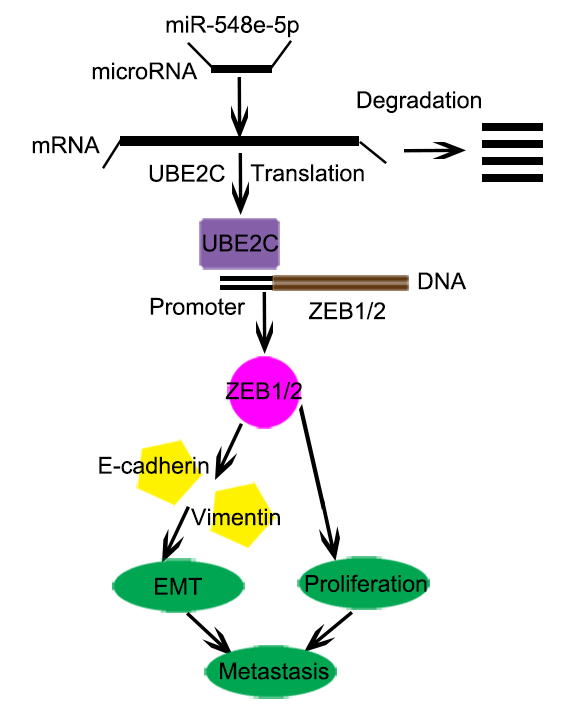
Results: We observed that 4,5-ubiquitin-conjugating enzyme E2C (UBE2C) expression was higher in NSCLC tissue than in the adjacent normal tissue and was associated with increased cell proliferation and invasion. UBE2C enhanced NSCLC progression and metastasis by affecting the cell cycle and inhibiting apoptosis. We also observed that miR-548e-5p was significantly downregulated in lung cancer tissue specimens, which decreased the expression of its direct substrate, UBE2C. Moreover, miR-548e-5p overexpression and UBE2C under-expression significantly suppressed lung cancer cell proliferation, migration, and invasion. Luciferase reporter and chromatin immunoprecipitation assays indicated that miR-548e-5p directly binds to the 3′-UTR of UBE2C and decreases UBE2C mRNA expression. Furthermore, UBE2C knockdown downregulated the mesenchymal marker vimentin and upregulated the epithelial marker E-cadherin. Bioinformatics assays, coupled with western blotting and luciferase assays, revealed that UBE2C directly binds to the 5′-untranslated region (UTR) of the transcript of the E-cadherin repressor ZEB1/2 and promotes EMT in lung cancer cells.
Conclusion: miR-548e-5p directly binds to the 3′-UTR of UBE2C and decreases UBE2C mRNA expression. UBE2C is an oncogene that promotes EMT in lung cancer cells by directly targeting the 5′-UTR of the transcript encoding the E-cadherin repressor ZEB1/2. miR-548e-5p, UBE2C, and ZEB1/2 constitute the miR-548e-5p-UBE2C-ZEB1/2 signal axis, which enhances cancer cell invasiveness by directly interacting with e EMT marker proteins. We believe that the miR-548e-5p-UBE2C-ZEB1/2 signal axis may be a suitable diagnostic marker and a potential target for lung cancer therapy.
Keywords: miR-548e-5p, UBE2C, ZEB1/2, cellular growth, EMT, lung cancer metastasis
 Global reach, higher impact
Global reach, higher impact